INTRODUCTION
Tobacco use is a major causative factor for cancer. Continued use of tobacco even after cancer diagnosis has been found to be associated with various poor prognostic factors, i.e. second primary cancer, all-cause and cancer-specific mortality, cancer recurrence, poor treatment response, and treatment-related toxicity1-4. The psychological impact of continued tobacco use adds to already prevailing depression in patients and overall to the family’s impaired functioning caused by the diagnosis of cancer5.
Time of cancer diagnosis has been termed as a ‘teachable moment’ for tobacco cessation, as diagnosis of cancer personalizes harms of tobacco use and directs priorities to restoration and maintenance of good health for patients and their loved ones using tobacco. On the other hand, severe nicotine dependence, urgency of cessation, fatalistic attitudes about cessation benefits, cancer-related psychological distress, treatment factors, and the presence of tobacco users in the social network have been identified as barriers to this ‘moment’6. Apart from diagnosis of cancer being a motivational factor to quit tobacco use, a conjunction with tobacco cessation programs that target these factors may improve quitting rates in patients. For these reasons smoking cessation interventions have been recommended as part of standard oncologic treatment7.
A few systematic reviews and meta-analyses assessing the efficacy of tobacco cessation intervention in cancer patients, in general8-10, as well as in head and neck cancer (HNC) patients, specifically11-13, have been conducted. The meta-analysis by Klemp et al.11 showed smoking cessation was achieved considerably more often in HNC patients who received cessation counselling compared with those receiving usual care. The majority of the literature on clinical impact of continued tobacco use after diagnosis has been on tobacco smoking. Among the randomized controlled trials assessing tobacco cessation in cancer patients, in general14-21, as well as in HNC patients specifically22-24, none has focused on patients using smokeless tobacco (SLT).
Intriguingly, significant changes in the attitudes and behaviours of patients’ relatives toward cancer prevention and screening occur after the patients are diagnosed with cancer25. However, Sarna et al.26 reported that even though higher quit rates were seen in cancer patients, quit rates were lower among family members who smoked. Expanding the benefits, therefore, to psychological interventions targeting tobacco use in relatives along with those diagnosed with cancer may be beneficial. Moreover, evidence suggests that continued smoking undermines patients’ individual efforts to quit and hence there is a need to consider the patient–family member dyad in the context of tobacco cessation27-28. Studies using dyadic involvement with targeted education of the family member regarding illness state and impact of smoking on health have found better patient engagement with quitline services29, overall better smoking cessation rates30 and increased supportive behaviour of the family members towards the patient31.
In the state of Chhattisgarh, India, the prevalence of tobacco-use disorders (29.9%) is above the national prevalence (20.9%)32, with 47.7% of men, 24.5% of women, and 36.0% of all adults, currently using SLT33. This is 5 times greater than rates of tobacco smoking. Notably, National Cancer Registry Programme (NCRP, 2012–14) reports 45.7% and 16.5% (43.4% and 13.2%, in the state of Chhattisgarh) of cancers to be tobacco related cancers, in males and females, respectively34. Of these 48.55% in males and 41.82% in females have head and neck cancers (HNC: lip, tongue, mouth, oropharynx, hypopharynx, pharynx, and larynx), which have been associated with use of SLT35. Primary objective of the proposed study is to assess the efficacy of a brief tobacco cessation intervention (compared to treatment as usual) on cessation rates of tobacco chewing in newly diagnosed HNC patients and their relatives. Changes in other patterns (such as amount, frequency, dependence etc.) and knowledge and attitudes (towards association of SLT and cancer, quitting, continued use, towards health warnings, health-behavioral modifications, long-term consequences on treatment etc.) towards tobacco chewing will be secondary outcome variables. An ancillary objective of the study is to find out the effect of diagnosis of HNC on cessation rates, patterns and attitudes towards tobacco chewing in patients and their relatives, and to determine predictors of post intervention, change in attitude and pattern towards SLT use. Here, we present the rationale for the study, the study design and its protocol.
METHODS
This current study protocol follows the SPIRIT 2013 Statement, which provides recommendations for a minimum set of scientific, ethical and administrative elements that should be addressed in a clinical-trial protocol.
Study design
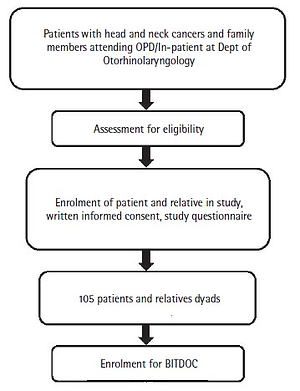
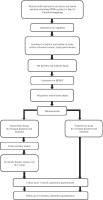
The study will be conducted in two phases. Phase 1 is a cross-sectional, descriptive, questionnaire-based study of patients and their relatives attending the outpatient/inpatient services. From the total number of participants recruited, a group of participants will be enrolled for the Brief Intervention for Tobacco when Diagnosed with Oral Cancer (BITDOC), i.e. the second phase. Phase 2 is a longitudinal, randomized control trial study. A flow diagram of the study design is presented in Figure 1. The study has the approval of the institutional ethics committee and has been registered at ctri.nic.in (identifier: CTRI/2019/02/017738).
Study settings
The study began in January 2019 at the All India Institute of Medical Sciences (AIIMS), Raipur, Chhattisgarh, India, which is a tertiary care centre offering speciality services to patients belonging to the states of Chhattisgarh, Odisha, adjoining regions of Maharashtra, Madhya Pradesh, and Jharkhand. The current study is being conducted by the Department of Psychiatry in collaboration with the department of Otorhinolaryngology. Sample will be recruited from outpatients attending the department of Otorhinolaryngology. The study is designed to be completed by one year from the start of the project.
Participants
The study will include patients recently diagnosed with head and neck cancers; with a history of current use (continued use for at least 6 months before the diagnosis of cancer) of smokeless tobacco, who are aged >18 years, and belong to either gender. Family members to be included in the study should have lived with the patient for at least one year. Family members who are currently pregnant or diagnosed with severe mental disorders like psychotic disorders, obsessive compulsive disorder, dementia, or with a past diagnosis and treatment for any cancer or precancerous lesions/conditions will be excluded from the study. Family members who are not SLT users will also be able participate in the study. The rationale being to assess attitudes towards SLT use and enhance them during the intervention. Support person (non-users) intervention has been found to be effective in promoting quitting in users29.
Sample size
Based on the findings of Koca et al.25, i.e. 37.4% proportion of the relatives having a change in their attitudes and behaviors toward prevention and screening of cancer, we calculated an a priori sample size. With a 25% relative margin of error, the sample size, n, required to estimate the given proportion was found to be about 105, calculated from the expression:
Where Z is the level of confidence at 95%, e is the tolerated margin of error and p is the estimated proportion of the population. Tentatively, in Phase 1, we intend to have an analysable sample of 105 patients and 105 relatives. For Phase 2, sample size calculation was done keeping 90% power and error probability of 5% (2-tailed), and effect size (d) of 0.345 (meta-analysis by Klemp et al.11). With correlation between the repeated measures of zero, the estimated total sample size required is 48. With an expected 10% dropout in the sample, total calculated sample size in each of the two groups is 53; hence, a minimum of 27 dyads of patients and relatives will be recruited.
Recruitment of participants
The participants in the study will be recruited using a purposive sampling method. All the participants recruited as part of Phase 1 will be approached to be part of Phase 2. For Phase 2, the participants will be randomized into two subgroups: A – BITDOC; B – ‘Treatment as usual (TAU)’. Blocked randomization with fixed block sizes of four will be used. A concealed allocation schedule using sealed opaque envelops will be undertaken for allocating patients in the two groups.
Intervention
Intervention will be delivered by trained mental health professionals having adequate experience in psychotherapy. The BITDOC shall be based on the principles of motivational interviewing36 and the 3As model37. The 3As (Ask cancer patients about their tobacco use; Advise patients about the benefits of quitting; Act to refer the patient to a smoking cessation program) model has been found to be suited for delivering tobacco treatment in primary care practice38. Further, a recent study has recommended this model for tobacco cessation in cancer patients7. The intervention shall consist of a 2-hour primary session and a 15-minute booster (refresher) session spaced over 10–15 days. The primary session will be conducted in an individual (dyad) therapy mode and the booster session will be in an individual format (telephonic mode may be used when in-person sessions not feasible). The contents of intervention will be as described in Table 1.
Table 1
Steps and components of the intervention
Outcome variables and their assessment
A study-specific questionnaire evaluating different variables as mentioned below has been developed for use in this study.
Independent variables
Pre-diagnosis and post-diagnosis pattern of SLT use: present/absent, types (betel quid with tobacco, khaini, gutka, oral tobacco application, pan masala with tobacco), amount, total duration, dependence (present/absent), duration in dependence pattern, severity of dependence, attempts at abstinence, period of abstinence, average daily cost, psychological co-morbidity (depression, anxiety, impaired quality of life, psychological distress) etc.
Pre-diagnosis and post-diagnosis ‘knowledge and attitude’ towards SLT use based on whether: it leads to cancer; they saw pictorial health warnings on packets; pictorial health warnings on packets appropriate; pictorial health warnings on packets have significant impact; the size of the pictorial health warnings on packets be enlarged; they saw/heard/read health warnings aired/printed in media; health warnings aired/printed in media appropriate; health warnings aired/printed in media have significant impact; the frequency of health warnings aired/printed in media be increased; attempted to quit SLT use; attempted to reduce SLT use.
‘Knowledge and Attitude’ towards long-term consequences with SLT, based on whether: it can aggravate cancer or cause recurrence; it can cause cancer elsewhere; it interferes with treatment; continued use can lead to earlier death; it can aggravate psychological co-morbidity (depression, anxiety, impaired quality of life, psychological distress); there is urgent need to quit tobacco; there is a further worth in quitting tobacco; benefits of quitting tobacco outweigh difficulties of quitting.
Attitude towards ‘health behavioural’ modifications: pattern of comorbid tobacco smoking and any other substances (similar to pattern of SLT); attitude change in eating habits, physical exercise, other lifestyle factors (stress, leisure, holidaying, shopping, spending time with family and friends); screening for cancer; use of alternative/complementary therapies and/or vitamins for protection against cancer etc.
Disease-related factors (only for patient): cancer site; stage (including precancerous stages); comorbidities; and treatment (surgery and radiation/chemotherapy, duration, side-effects, prophylaxis).
Outcome variables shall be applied before (1–3 days) the commencement of intervention (assessed as part of ‘impact of cancer diagnosis’ phase), at one and three months after the intervention. Principally, the follow-up assessments shall be conducted in person. However, when not feasible, assessments over telephone conversations shall be carried out. Outcome assessments shall be blinded to the intervention group allocation and conducted by the person collecting data in the ‘impact of cancer diagnosis’ phase.
One person (research assistant) shall rate the outcomes and he/she shall be blind to randomization/allocation schedules. Person providing the intervention will not aid any outcome assessments.
Assessment tools/Instruments
A study-specific questionnaire will be used for assessment and evaluation of patients and their relatives in the study. The questionnaire will be based on variables assessed across various relevant studies and other specific domains identified in the literature review.
Study specific questionnaire
SLT (type, duration, amount): Global Adult Tobacco Survey 2 (GATS 2)33;
Severity of tobacco dependence: A modified version of Fagerström developed to assess severity of dependence of smokeless tobacco will be used39-40;
Attitude towards health warnings: Adopted and extended based on Ahsan et al.41;
Change in attitudes towards health-behavior modifications (substance, physical exercise, food habits, lifestyle etc.) towards cancer prevention and screening (Koca et al.25);
Knowledge and attitude towards long-term consequences with SLT related to cancer progression and management (McBride and Ostroff6, Choi et al.2).
Tumour stage (0–IV)
Tumor stage will be assessed using the American Joint Committee on Cancer (AJCC) staging classification system42.
Comorbidities
Comorbidities will be assessed by the Adult Comorbidity Evaluation-27 (ACE-27) and classified as none, mild, moderate, or severe43.
Statistical analysis
Frequencies and descriptive statistics shall be calculated and compared using independent samples t-test or chi-squared test. Pearson/Kendall tau correlation coefficients and corresponding partial correlation coefficients shall be calculated for correlation assessment. A step-by-step discriminant function analysis using Wilk’s lambda method shall be used for prediction analysis. A 3×2 repeated measures ANOVA will be used to analyse the pre-post effects of the interventions on the outcome variables. ‘Both patient-relative use SLT’ and ‘only patient uses SLT’ covariation within the dyads will be corrected in the analysis.
DISCUSSION
In middle-income countries, like India, it is prudent that studies are conducted to assess the attitude of population towards various tobacco products, especially SLT which has a higher prevalence compared to other forms. It is also important to test various models that are cost effective, less intensive and addressing various barriers for promotion of tobacco cessation. Hence, a study like this is of utmost importance. This will help evaluation of not just the knowledge and attitude towards SLT in a population that has faced adverse consequences from the use to SLT but also for assessment of changes brought about by diagnosis of HNC. This study will further help in creating a model that can be used for promotion of smoking cessation in such populations.
Limitations
The sample size estimation for the randomized trial phase of the study is based on effect size of interventions using CBT and counselling, with most of the studies including NRT also, for smoking cessation and hence it is an extrapolation. Therefore, effect sizes used for sample size estimation in the study may be overestimated.
More importantly, use of the 3As model might limit the individual efficacy compared to other models such as CBT, 5As etc., especially with regard to high rates of tobacco use and potential lack of knowledge and negative attitudes towards tobacco cessation. However, keeping the resource limited (in terms of a very large mental health treatment gap)32 settings in India, we believe the brief intervention model using 3As should fare well in terms of feasibility for use on community scale.
CONCLUSION
This is a first study in India for the evaluation of a brief intervention for tobacco cessation in cancer patients. There is a need for conducting such studies at a larger scale for evaluation of effectiveness of brief intervention in populations at risk. If effective, this model may bring about expected changes towards tobacco cessation without needing intensive therapy, especially in places where there are fewer trained clinicians and specialists.