INTRODUCTION
In an era where traditional tobacco smoking is experiencing a decline, e-cigarette usage is dramatically increasing, attracting both former tobacco smokers and never smokers1, 2. Although e-cigarette use has been widely reported to be experimented with as a smoking cessation tool, it still lacks the scientific evidence to be considered as one3.
E-cigarette heats and vaporizes a nicotine-containing liquid, which is delivered fast to the respiratory and central nervous system. While vaporized e-cigarette aerosol may contain fewer toxicants than conventional cigarette smoke, studies evaluating the e-cigarettes health effects and safety are still ongoing.
However, in cell lines, e-cigarette vapour increases oxidative stress, inflammation4, 5, may influence susceptibility to virus infection6 and induces DNA strand breaks and cell death7 on airway epithelial cells. It is also cytotoxic, induces apoptosis in fibroblats8, initiate inflammation at Kupffer9 and enthothelial10 cells, and alters vascular smooth muscle cells11. There is also an increasing number of studies in animal models implicating possible health effects. Namely, exposure to e-cigarette vaping results in immunomodulatory effects that are similar to those observed after exposure to conventional cigarette smoke, impairs pulmonary anti-bacterial and anti-viral defenses12, aggravates allergen-induced airway Inflammation and hyper-responsiveness13, induces toxicity, and oxidative stress.
The aforementioned studies indicate that e-cigarette use might have significant consequences on the human respiratory system. However, scientific data on health effects of e-cigarettes in humans are impressively limited. There are scarce case reports showing e-cigarette effects on the respiratory system14–5, and a few studies showing acute effect in aortic stiffness and blood pressure16–7. Although there are numerous studies addressing the effects of traditional cigarette18–22 in respiratory physiology, very few are available concerning the same topic for e-cigarettes23–55. Vardavas et al reported an increase in lung resistance, impedance, and a decrease of FeNO23. Contrastingly, Schober et al and Marini et al reported a resultant increase of FeNO24. An amelioration of respiratory symptoms and lung function was reported after switching from regular cigarettes to e-cigarette in a small group of asthmatic smokers26.
Our study is designed: a) to investigate the acute effects of e-cigarette use in respiratory systems of smokers with obstructive airways diseases (Chronic Obstructive pulmonary disease (COPD) and asthma), “healthy” smokers, and healthy never smokers, and b) to compare the effect of a nicotine e-cigarette (11mg) to a nicotine-free one in healthy non-smokers.
METHODS
Study Population
Seventy-six (76) adults participated in our study. Fifty-five (55) subjects were smokers recruited from our smoking cessation clinic (16 with COPD, 11 with asthma, 27 with no respiratory history) and 21 healthy nonsmokers. (nine of which used an e-cigarette containing 11mg nicotine and 12 a nicotine free e-cigarette). In this manuscript, we use the term “healthy” to connote those without any history and/or symptoms of any overt airway disease. All participants were naïve e-cigarette users but, were able to vape successfully during a pretest training.
Inclusion criteria for smokers were: a) age>18 years, b) smoking habit > 15 cigarettes/day, c) No previous or current smoking cessation treatment, d) no history of exacerbations during last month, and e) ability to cooperate with the applied techniques for detecting airways inflammation and lung function testing. Exclusion criteria were: a) any bacterial or viral illness for the last 4 weeks b) ex-smoking, b) other acute or chronic respiratory disease, but asthma and COPD, c) chronic heart, liver, renal disease, or other serious disease, d) drug abuse, and e) pregnancy, lactation, malignancies, autoimmune, and immunodeficiency conditions.
Study protocol
Participants visited the lab 1-2 weeks after recruitment to complete the main protocol procedures. Food or drink consumption was not allowed for at least two hours before study measurements. Smoking abstinence was advised for at least 6 hours before measurements. No bronchodilators were taken for the previous 24 hours. The hospital’s Ethics Committee approved the study protocol and all participants obtained informed consent.
Respiratory symptoms, vital signs, airways non-invasive inflammatory markers (exhaled NO and breath airways temperature), lung function test (airway resistance, specific airway conductance, single nitrogen breath test) were measured before and immediately after 10 min of ad lib use of an e-cigarette. The number of puffs was not statistically different among smokers and never smokers. However, never smokers who used the nicotine-free e-cigarette reported a greater number of puffs. The same brand of a first generation e-cigarette popular at the national level in Greece, was obtained from the same source, and used by all participants. The e-cigarette was recharged and refilled, according to the manufacturer’s instructions, with the same commercially available flavorless liquid.
Clinical symptoms (cough, sore throat, dry mouth, eye irritation, palpitations, and nausea), vital signs (heart rate, oxygen saturation), pleasure feeling and the total number of puffs were recorded for each participant.
Airway inflammation assessment was performed by measurements of non-invasive exhaled markers - exhaled breath temperature (X-halo breath thermometer X-halo, Delmedica, USA) and exhaled NO (Niox-MINO analyzer: Aerocrine AB. Aerocrine AB, P.O. Box 1024,SE-171 21 Solna, Sweden)27
Lung function tests were also performed before and after 10 min of the e-cig use. Simple spirometry was performed with the Vmax apparatus (Vmax Encore 22, SensorMedics, Yorba Linda, CA, USA) using the “fast maneuver”. The diffusing capacity for carbon monoxide (DLCO) was also determined by the single breath technique (Vmax Encore 22). Static Lung Volumes were determined by body plethysmography (Vmax Autobox Plethysmograph, SensorMedics, Yorba Linda, CA, USA). Predicted values of spirometry, static lung volumes and DLCO were from the European Community for Coal and Steel28. Airway resistance (Raw) and specific airway conductance (sGaw) were also assessed by the body plethysmography (Vmax Autobox). Predicted values were from Dubois et al29. The means of two or more acceptable tracings for each subject were reported.
Finally, the single breath nitrogen test (SBN2T) was performed (Vmax Encore 22) for the assessment of the slope of phase III in order to evaluate small airways function. Subjects were asked to breathe to residual volume (RV) and immediately perform a slow maximal inspiration of oxygen followed by a slow (~0.5 l/s) expiration to RV. Phase III slope was calculated as the best-fitted line through phase III. The reported values (DN2/L, % pred) of phase III slope are means of two or more acceptable tracings. Normal values for phase III slope were from Buist and Ross1,30.
Statistical analysis
Data are expressed as mean ±SD, unless otherwise stated. For comparisons between groups Student’s paired t-test, Student’s t-test, Mann-Whitney rank sum test, Chi-square and Student’s one-way ANOVA with Holm-Sidak’s correction were used. A p≤0.05 value was considered as significant. Statistics were performed using the SigmaStat V3.5 and SigmaPlot V10.0 statistical software (Jandel Scientific, CA, USA).
RESULTS
Demographic characteristics of the study population groups and baseline spirometry data (FVC, % pred, FEV1 % pred FEV1/FVC, %) and DLCO, %pred are shown in Table 1. There were no differences between the groups of healthy smokers and never smokers, who did not differ by age, gender, and BMI. In contrast, COPD smokers were older, had a higher pack-years history and lower baseline spirometry values compared to the other smokers.
Table 1
Study population characteristics and baseline lung function.
Impact on symptoms
Clinical symptoms reported immediately following 10 min of e-cig use are shown in Table 2. The majority of healthy smokers, and never smokers using e-cigarette with nicotine concentrations predominantly reported a dry mouth and sore throat. Acute cough was reported by 69% of smokers with COPD, 55% of smokers with asthma, “healthy” smokers (68%), and healthy never smokers following both use of 11mg and 0 mg nicotine concentrations (44% and 50%, respectively). Palpitations were reported by 46% of asthmatics using nicotine containing e-cigarette. Lower percentages of palpitations were reported by COPD patients (19%), healthy smokers (36%) and healthy never smokers (33%). Nausea was reported (below 50%) in all groups after e-cig use.
Table 2
Self-reported Symptoms reported post use of a single e-cigarette containing 0mg/11mg of nicotine.
Effects on vital signs and airway inflammation
Vital signs and airway inflammatory markers are shown in Table 3. The 11mg nicotine e-cigarette caused a statistically significant (p<0.05) increase in heart rate in all subjects, except COPD patients. No changes in heart rate were detected following the use of the nicotine free device in non-smokers. No changes in arterial blood pressure were observed in all participants. A small but statistically significant decrease in oxygen saturation (SpO2) was noted following e-cigarette use in “healthy” smokers (p<0.001) and COPD smokers (p<0.05). Exhaled NO decreased following e-cigarette use in all subjects, but these changes were not statistically significant. Similarly, no statistically significant changes were noted in exhaled breath airway temperature.
Table 3
Vital signs and markers of airway inflammation. Pre- and post-use of an e-cigarette
| Nicotine=11mg | Nicotine=0mg | ||||
|---|---|---|---|---|---|
| COPD smokers (n=16) | Asthma smokers (n=11) | Smokers (n=28) | Non smokers (n=9) | Non smokers (n=12) | |
| SpO2 | |||||
| Pre | 96.4±1.9 | 97.2±1.53 | 98.2±1 | 97.4±2.1 | 98.4±0.9 |
| Post | 95.8±1.8* | 96.6±1.36 | 97.1±1.5** | 97±0.7 | 98.4±0.5 |
| HR | |||||
| pre | 76.0±11.2 | 84.9±14.2 | 75.2±9.8 | 79.1±13.8 | 80.4±9.3 |
| Post | 79.4±13.7 | 93.5±15.1* | 92.1±16.9** | 92.2±17.4* | 78.8±9.0 |
| eNO | |||||
| Pre | 12.7±6.4 | 12.1±4.8 | 11.7±10.2 | 16.0±4.5 | 13.3±8.3 |
| Post | 11.7±8.2 | 10.6±4.6 | 10.7±10.2 | 14.7±4.6 | 10.8±6.5 |
| Air Temp | |||||
| Pre | 33.8±0.9 | 33.4±1.1 | 33.7±1.5 | 34±1.5 | 33.1±2.0 |
| Post | 34.2±1.5 | 33.5±1.2 | 34.1±0.7 | 33.9±1.9 | 32.7±3.1 |
Changes in Lung Functions
Lung function changes are presented in table 4. Baseline measurements (before e-cigarette use) showed an increased mean values of airways resistance (Raw) and decreased values of specific airways conductance (sGaw) in smokers with COPD and asthma compared with healthy smokers and never smokers. Following 10 min use of e-cigarette dynamic static lung volumes, and DLCO showed no statistically significant changes in all participants. However, there were significant changes detected in airways resistance (Raw) and specific airways conductance (sGaw) noted after e-cigarette use. A significant increase in airways resistance (ΔRaw) was detected in asthmatic (p=0.034) and healthy smokers (p=0.004). Strikingly, an increase in Raw was detected in never smokers after e-cigarette use both when nicotine was contained (p<0.005) or not (p<0.001) (Fig .1)
Figure 1
Airway resistance pre- and post-inhaling the content of a single e-cigarette. Box plot graphs for each of the groups tested. The end of the boxes define the 25th and 75th percentiles, with a line at the median and error bars defining the 10th and 90th percentiles.
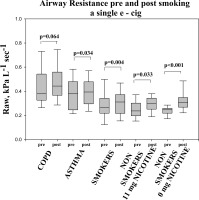
Table 4
SBN2 data, airway resistance (Raw), and special airway conductance (sGaw) pre- and post-use of an e-cigarette
| Nicotine=11mg | Nicotine=0mg | ||||
|---|---|---|---|---|---|
| COPD smokers (n=16) | Asthma smokers (n=11) | Smokers (n=28) | Non smokers (n=9) | Non smokers (n=12) | |
| DN2/L, %pred | |||||
| Pre | 299±182 | 141±52 | 98±35 | 92±39 | 88±25 |
| Post | 316±185 | 172±65* | 106±29 | 97±38 | 99±22 |
| Raw, kPa L−1 sec−1 | |||||
| pre | 0.43±0.18 | 0.38±0.13 | 0.29±0.12 | 0.25±0.07 | 0.24±0.04 |
| Post | 0.47±0.17 | 0.40±0.11* | 0.31±0.13* | 0.29±0.06* | 0.32±0.08** |
| SGaw, sec−1 kPa−1 | |||||
| Pre | 0.54±0.19 | 0.84±0.31 | 1.16±0.47 | 1.31±0.22 | 1.20±0.27 |
| Post | 0.52±0.19 | 0.80±0.33 | 1.03±0.40* | 1.11±0.18* | 0.95±0.18* |
A decrease in specific airways conductance (sGaw) was also detected in all groups but this change was statistically significant only in healthy subjects, both smokers (p<0.05) and never smokers (p<0.05). In healthy non-smokers, this decrease occurred using both nicotine and nicotine-free e-cigarette (Fig 2). sGaw was the only test were gender differences were found between “healthy” smokers.
Figure 2
Specific airway conductance pre- and post-ecigarette use depicted withbox plot graphs for each of the groups.
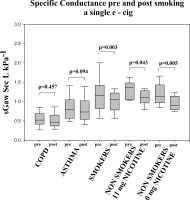
Changes in Raw and sGaw were not statistically significant in smokers with COPD.
Evaluation of the small airways function by the single breath nitrogen test resulted in an increase of the slope of phase III curve (expressed as ΔN2/L, % pred) following e-cigarette use in all groups. The slope of phase III curve was already increased at baseline measurements in both COPD (p<0.001) and asthmatic (p<0.05) smokers compared with healthy smokers. After use of the e-cigarette the slope of phase III curve significantly changed only in asthmatic smokers (p<0.01). A simple explanation for the latter is that dysfunction of asthmatic smokers’ small airways was less than this of COPD patients. It could also be a sign of increased bronchial hyperactivity since research in animal models has already showed that e-cigarette aggravates allergen-induced airway Inflammation and hyper-responsiveness13.
DISCUSSION
The assessment of the acute effects of e-cigarette use on the respiratory system and airway physiology in non-healthy (COPD, Asthma) smokers, “healthy” smokers, and never smokers has not been the focus of extensive research so far. Our study exposes the effect of short-term use of e-cigarette on respiratory symptoms, vital signs, airway inflammation and lung function by specifically assessing airway physiology in smokers with asthma and COPD, “healthy” smokers, and healthy never smokers.
Our outcomes are in accordance with a previously published study on short-term effect of e–cigarette use on airways resistance23. The take home message is that short-term (10 min) use of an e-cigarette has an acute effect on the respiratory system in smokers -a significant increase in airway resistance (Raw). The effect was more profound in both asthmatic and “healthy” smokers. More striking is the increase in Raw in healthy never smokers despite using an e-cigarette without nicotine.
Against the backdrop of other studies, the single breath nitrogen test to further assess the effect of e-cig on small airways was used for the first time to measure and capture short term effect of e-cigarette both in smokers with COPD and asthma. The slope of phase III of the single breath test is used as an index of small airway dysfunction and distribution of ventilation. It has been previously shown that the baseline slope of phase III is increased higher than normal in patients with COPD32. Our result reaffirms the increased slope of phase III in COPD and the normal slope of “healthy” smokers and non-smokers cited in the aforementioned study32. The significant increase immediately after the use of e-cigarette in the slope of phase III experienced only in asthmatic smokers might reflect early small airways impairment and deterioration of homogeneity of ventilation in this population.
In contrast with previous studies, we did not detect significant changes in FeNO5,23. This may be due to the short time of exposure or the small number of observations.
Interestingly, lung function impairment, as reflected in Raw and sGaw, was demonstrated regardless of the nicotine concentration of the e-cigarette. As animal models have indicated negative effects after exposure to e-cigarette liquids containing aromatics5,33, it would be of interest to test the effect of these constituents in humans in future studies.
We identified that short-time e-cigarette use led to participants cough regardless of nicotine concentration. These responses probably represent vagal mediated protective reflexes as they promote proximal deposition of vaporized particles and their subsequent ejection from the lungs by cough and mucociliary clearance. Our results are in line with previous studies showing that e-cigarettes may produce mouth, throat irritation, and dry cough, a factor that may be moderated however by the user’s experience in vaping8,36.
Palpitations were reported by participants who used the nicotine containing e-cigarette reflecting nicotine’s properties. However, a smaller number of COPD patients vs. other groups reported palpitations caused by the e-cigarette. This might be explained by the fact that the COPD patients had a higher number of pack years compared to other groups in our study. Most of studies addressed the effect of e-cigarette in heart rate, blood pressure or heart beat instead of palpitations. Increased heart rate is a common finding7,16. Following short time e-cigarette use, a statistically significant decrease in oxygen saturation (SpO2) was noted in COPD patients who had already a lower baseline level compared with the other smoker groups. Therefore, increased awareness should be raised concerning blood oxygen desaturation and tachycardia caused by long-term e-cigarette use in susceptible populations (i.e. COPD) with hypoxia and/or cardiac arrhythmias.
There is significant debate around the individual and population implications of e-cigarette use39–45.
Our study is not without limitations. Foremost, our participants are all naïve vapers. Although they were recruited after a pretest training, little differences in vaping are apparent. Moreover, we used a first generation e-cigarette. It will be interesting to see if our results can be replicated with newer generation’s e-cigarettes in future studies. Although the e-cigarette used was popular at the national level and purchased from the same source, variability of nicotine concentrations and liquid ingredients cannot be excluded. Lastly, a larger sample size, inclusion of testing all e-cigarette ingredients, a newer generation e-cigarette, chronic use and experienced users, would be interesting to be addressed in future studies as they could add important information to our findings. Further research is needed towards these directions.
CONCLUSIONS
This study has shown that short-term use of e-cigarette has an acute effect on the respiratory system. Smokers with obstructive airways disease and mainly smokers with asthma may exhibit deterioration of respiratory symptoms and lung function impairment following use of an e–cigarette. According to our study, effects on airways could not be attributed to nicotine. Further studies are needed to unfold the short and long-term health effects of e-cigarettes to support the decisions of policymakers, health care providers, and consumers.

