INTRODUCTION
An electronic cigarette (e-cigarette) is an umbrella term for an electronic device that delivers usually nicotine and/or other products, including solvents and flavorings, to the user. It is generally accepted that e-cigarettes were introduced to Europe in 2006 and to the US in 2007, and as of 2017, 433 brands of e-cigarettes and 15586 flavored e-liquids were on the market1. Although there are variations in the appearance of e-cigarettes, the National Institute on Drug Abuse has reported that regardless of their design and appearance, the devices generally operate in a similar manner and are made of similar components2. However, variations in device design and user practices mean that, as an intervention, e-cigarettes provide heterogeneous exposure to nicotine and other products delivered to the user3-5.
The emergence of e-cigarettes is a disruptive change with inter-related implications for both public health policy and day-to-day clinical practice6-8. Against a context of debate on the relative harms, benefits and uncertainties9, countries are adopting shifting and diverse approaches to e-cigarette regulation in the context of their tobacco control policy10. While healthcare professionals report differing views on their role in smoking cessation for patients, some express concern about risks at individual and population level11.
Scientific evidence is essential to support both policy-makers and healthcare professions navigate and resolve difficult questions about e-cigarettes for public health and individual patient care12,13. Systematic reviews are one critical tool in formulating recommendations in clinical guidance, and can also be mobilized to inform discussion and decision in the policy arena14,15. While the debate on e-cigarette is complex16, establishing the effectiveness of e-cigarettes as a smoking cessation aid is a central question which is especially amenable to systematic review.
Several systematic reviews have been published in this area17-24, however, the results of these reviews have been contradictory and new RCT evidence has emerged since many of these reviews were undertaken.
This systematic review evaluated the efficacy and safety of e-cigarettes in helping people who smoke to achieve abstinence at 24–26 and 52 weeks, compared with electronic non-nicotine delivery systems (ENNDS, no nicotine), or any comparator recommended for smoking cessation treatment or combination of treatments using randomized controlled trials (RCTs).
METHODS
We used systematic review techniques that involved searches of three databases (Ovid MEDLINE, Wiley Cochrane Library, and Elsevier Embase), in February 2020. These searches were updated in May 2021. The version of Embase used changed from Elsevier Embase in 2020 to Ovid Embase in 2021, which may explain the increased return from the Embase search in 2021. Controlled vocabulary was updated for each database (MeSH, EmTREE). The 2021 Ovid MEDLINE search strategy is given in the Supplementary file Appendix 1. We searched for RCTs of e-cigarettes compared with ENNDS (no nicotine), or any comparator recommended smoking cessation treatment or combination of treatments (Table 1). Our primary outcome was smoking cessation – continuous abstinence without relapse throughout the follow-up period – as defined by European Medicines Agency guidance25. We extracted this endpoint measured at 6 months (24–26 weeks) or 1 year (52 weeks) after treatment initiation. These endpoints are consistent with the Russell Standard on endpoints in trials of smoking cessation interventions26 and is consistent with current expert opinion27. We also extracted adverse events from identified RCTs. Completed in line with agreed systematic review principles were: two-person independent screening using the inclusion and exclusion criteria presented in Table 1; two-person independent assessment of bias using the Cochrane Risk of Bias (Version 2) tool28; formal extraction of data into a bespoke form with verification by a second person; feasibility assessment to decide if meta-analysis was appropriate following published guidance29,30, and network meta-analysis (NMA) of data using the gemtc package in R version 3.6.0 where feasible.
Table 1
Eligibility criteria
For this analysis, we selected NRT as the reference treatment because it is a common control treatment in the RCTs identified, which is a common standard of care in international clinical guidelines for treatment of tobacco dependence31. Laboratory verified data on smoking abstinence were preferred, but self-reported data were also included.
Before the analysis was undertaken, an assessment was conducted to assess the feasibility and appropriateness of undertaking an NMA29,30. The feasibility assessment considered population, interventions and comparators, outcomes and their endpoints, and risk of bias.
Application of NMA for evidence synthesis has become widespread, and it has been used, for example, in the Cochrane review of pharmacological interventions for smoking cessation32 . We used NMA to compare the multi-arm treatments at 24– 26 weeks in a single analysis by combining direct and indirect evidence in a single network. NMA is a next-generation evidence synthesis tool which can better serve decision-making than traditional pairwise meta-analysis through mobilizing a wider body of direct and indirect evidence across trials and improving estimate precision33,34. We used the gemtc package35 in R version 3.6.036 to conduct an NMA of smoking cessation for e-cigarettes (electronic nicotine delivery system abbreviated to ENDS) as it allows for arm-based trial data to be analyzed and the package can summarize the comparative treatments effects as random effects relative risks. We specified the following parameters: 250000 ‘burn-in’ iterations to be discarded, 500000 iterations for analysis, and three separate chains. Diagnostic tests were run to check model convergence. Thinning of the chains was specified to reduce the risk of autocorrelation. Default priors as specified by the gemtc package were used.
A key assumption of NMA is that of evidence consistency that is, that estimates of treatment effects from direct and indirect evidence agree. We employed the Dias et al.37 suggestion that the standard consistency model be compared with an inconsistency model. We have compared the main model (consistency model) against an inconsistency model that assumes unrelated mean (relative) effects using a function of the gemtc package35. We also compared the direct head-to-head meta-analysis results versus the NMA outputs to further check for potential inconsistency. Meta-analyses for the direct comparisons were run using the metagen function in the meta package for the R programming language37,38.
RESULTS
Ten RCTs reported in 16 publications39-54 met the inclusion criteria for efficacy (N=8)39,40,45,47-49,51,54 and safety (N=10)39,40,45,47-49,51-54 of ENDS in smoking cessation. Two trials reported safety data only52,53. The PRISMA flow chart is presented in Supplementary file Appendix 4. Pertinent characteristics of the included RCTs are provided in Table 2.
Table 2
Country, sample size, and comparators of included primary RCTs
| Study ID | Primary publication | Secondary publication | Participants | Country | Comparator 1 | Comparator 2 |
|---|---|---|---|---|---|---|
| ASCEND | Bullen et al.39 2013 | - | 657 | New Zealand | NRT monotherapy: patches (21 mg) | Placebo e-cigarette |
| ECLAT | Caponnetto et al.40 2013 | Campagna et al.41 2016 | 300 | Italy | Placebo e-cigarette | - |
| Cibella et al.42 2016 | ||||||
| Farsalinos et al.43 2016 | ||||||
| Russo et al.44 2016 | ||||||
| TEC | Hajek et al.45 2019a | Hajek et al.46 2019b | 886 | UK | NRT combination: participants’ choice (no dose specified) | - |
| Halpern 2018 | Halpern et al.47 2018 | - | 2012a | USA | No additional treatment (apart from counselling) | - |
| Lee 2019 | Lee et al.48 2019 | - | 150 | South Korea | NRT monotherapy: gum (2 mg) | - |
| BETOFREE | Masiero et al.49 2019 | Lucchiari et al.50 2020 | 210 | Italy | Placebo e-cigarette | No additional treatment |
| Holliday 2019 | Holliday51 2019 | - | 80 | UK | No additional treatment | - |
| Hatsukami 2019 | Hatsukami52 2019 | - | 152 | USA | NRT monotherapy: gum (2/4 mg) | - |
| Lee 2018 | Lee53 2018 | - | 30 | USA | NRT monotherapy: patches (14/21 mg) | - |
| Eisenberg 2020 | Eisenberg55 2020 | - | 376 | Canada | Placebo e-cigarette | No additional treatment |
The number of participants in the RCTs ranged from 30 to 2012, with a median of 255 participants. Of the ten trials, two were based in Italy40,49, two in the UK45,51, three in the USA47,52,53 and one each in South Korea48, Canada54 and New Zealand39. Supplementary file Appendix 5 presents the detailed characteristics of the primary studies.
Smoking cessation data reported at 24–26 weeks39,40,45,47-49,51,54 and 52 weeks40,45,47 were extracted. Supplementary file Appendix 6 presents individual RCT results for smoking cessation at 24 or 26 weeks, and at 52 weeks. When extracting data from the articles for inclusion in the systematic review and meta-analysis, self-reported data, which were then biochemically verified (measuring cotinine or carbon monoxide levels), were preferred over self-reported unverified data. Biochemical verification of abstinence increases scientific rigor and is recommended in clinical trials of smoking cessation55. Data at 24–26 weeks were considered separately from 52 weeks data as results varied considerably between the studies that reported at both timepoints40,45,47 and there was a notable number of dropouts in all included trials across time (Supplementary file Appendix 7).
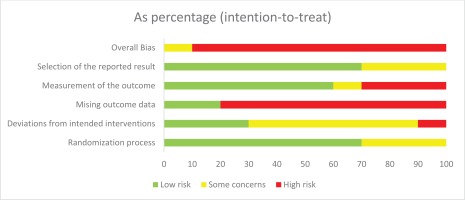
Risk of bias was assessed using the Cochrane Risk of Bias (Version 2) tool for trials28. Of the ten RCTs, nine were rated high risk of bias39,40,47-49,51-54 and one was rated as having some concerns45. These ratings were mainly driven by loss to follow-up and missing outcome data. The numbers lost to follow-up were high in all RCTs, and the proportions of successful cessation events were low in the RCTs (Supplementary file Appendix 7), both of which introduced uncertainty to the results of this systematic review. Participants who were lost to follow-up were treated as treatment failure in the RCTs and in this analysis. The number lost to follow-up was greater than 20% at 6 months in all trials, except one48, and was up to 30.3% in the study with the highest dropout rate52. Eight of the RCTs reported the numbers lost to follow-up, seven of these reported it separately by arm, and in six trials the number lost-to-follow-up was highest in the NRT arm; the seventh trial did not have an NRT arm and the number lost-to-follow-up was highest in the no additional treatment arm. The TEC trial was the only included study that attempted to assess the impact of missing data in four sensitivity analyses45. The risk of bias assessment is summarized in Figure 1.
During the meta-analysis feasibility assessment for the smoking cessation endpoint, we found that the study populations of the identified RCTs were comparable for pooling. One possible outlier population was the Halpern et al.48 2018 trial, which may have involved light smokers (Supplementary file Appendix 3), and therefore a sensitivity analysis was conducted to assess the impact of this trial on the NMA results. The feasibility analysis also considered the main intervention, which was the type of ENDS and dosage of nicotine given to the participants. Only first- and second-generation ENDS were included. ENDS was the intervention in all trials, and it was compared with NRT, or ENNDS (no nicotine), or no additional treatment. The nicotine dose in the NRT arm was most commonly monotherapy and some studies included more moderate dosing such as 2 mg gum and 14 mg patches (Table 2). The nicotine dose in ENDS arms ranged 5.4–18 mg/mL with the exception of the Lee49 2019 trial which reported a dose of 0.01 mg/mL. A second sensitivity analysis was conducted excluding the trail of Lee49. Seven55 of the eight cessation RCTs were at high-risk of bias. All eight RCTs reported smoking cessation results at 24 or 26 weeks. Seven of the eight RCTs verified the self-reported data with biochemical analysis55, the study which did not use biochemical verification was excluded in the third sensitivity analysis.
Cessation at 24 or 26 weeks
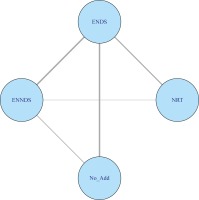
Figure 2 presents the data and network relationship using the eight RCTs that measured smoking cessation at 24–26 weeks39,40,45,47-49,51,55.
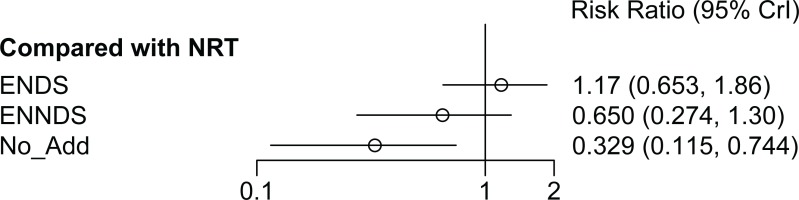
There was no statistically significant difference (evaluated using relative risk, RR, with 95% credible interval, CrI) between the ENDS and the NRT groups (RR=1.17; 95% CrI: 0.65–1.86) for smoking cessation at 24 or 26 weeks (Figure 3). There was no statistically significant difference between ENNDS (no nicotine) and NRT in achieving smoking cessation at 24 or 26 weeks (RR=0.65; 95% CrI: 0.27–1.30). No additional treatment is less effective than NRT and this result is statistically significant (RR=0.33; 95% CrI: 0.12– 0.74). The meta-analysis feasibility assessment identified heterogeneity in ENDS type and nicotine dose and therefore a random-effects NMA model at 24–26 weeks was reported.
Three sensitivity analyses were carried out. The first excluded the RCT which appeared to include lighter smokers47, the second excluded the RCT which had not biochemically verified their cessation data at 24 weeks45, and the third excluded the RCT with a lower dose of nicotine in the ENDS arm48. The results of these sensitivity analyses indicate that the main analysis was robust to assumptions relating to the smoking history of participants (NRT vs ENDS: RR=1.18; 95% CrI: 0.66–1.82 vs RR=1.17; 95% CrI: 0.65–1.86) and inclusion of unverified data (ENDS vs NRT: RR=0.93; 95% CrI: 0.46–1.93 vs RR=1.17; 95% CrI: 0.65–1.86), and lower nicotine dose (NRT vs ENDS: RR=1.35; 95% CrI: 0.73–2.34 vs RR=1.17; 95% CrI: 0.65–1.86). There was no change to statistically significance in any sensitivity analysis, all continued to demonstrate no statistically significant difference between ENDS and NRT. The complete sensitivity analysis is presented in Supplementary file Appendix 8.
The consistency and inconsistency models produced very similar estimates of treatment effect, agreeing in terms of direction and magnitude of effect. The Deviance Information Criterion was marginally lower for the consistency model (27.2 vs 28.9), indicating that the consistency model was appropriate. There was no difference in the direction of effect, from the direct and indirect evidence, for any of the comparisons. There is no evidence of inconsistency in the treatment effects presented in NMA at 24–26 weeks.
Cessation at 52 weeks
We did not undertake an NMA of smoking cessation at 52 weeks due to limited data; only three RCTs reported data for this timepoint40,45,47. Each study compared ENDS to a different control, and RCT results were mixed. One study with 300 participants found that ENDS appears more effective than ENNDS (no nicotine) for smoking cessation (RR=2.75; 95% CrI: 0.97–7.76), but the difference was not statistically significant40. One study showed that ENDS appears more effective than no additional treatment at 52 weeks (RR=6.11; 95% CrI: 0.33–113.24), but the difference was not statistically significant and the confidence intervals were very wide indicating a very small number of successful incidences of cessation47. One trial (N=886 participants) found that ENDS was more effective than NRT (RR=1.83; 95% CrI: 1.30–2.58), and this difference was statistically significant45. The substantial uncertainty for all of these analyses40,46,47 is attributable to the low number of successful events in each study coupled with the large numbers lost to follow-up (Supplementary file Appendix 6).
Adverse events
This systematic review found that standardized definitions were used to collect data on adverse events in two39,45 of the ten included RCTs. Only one of these specified the coding guidelines, Medical Dictionary for Regulatory Activities (MedDRA), that were used to classify the adverse events45. The adverse events in this study were all collected over a short period of time (≤12 months). A meta-analysis was not carried out as adverse events were not consistently reported, or classified.
European Medicines Agency classified adverse events were reported in nine RCTs 39,40,45,48,49,51-54 and were recorded at 8 months52,53, 12 months48,54, 6 months39,49,51,54, or 52 weeks40,45.
Documented adverse events included vital signs40,49,52 in three of the RCTs, and indicators of cardiovascular45,49,53 or psychiatric events40,45,49,54 in four of the ten RCTs (Supplementary file Appendix 9). Respiratory events (including shortness of breath and cough)40,45,48,49,52-54 and addiction associated potential and withdrawal events39,40,45,48,49,51 were documented in six of the ten RCTs each (Supplementary file Appendix 9). For all reported adverse events, the incidence of documented adverse events was lower in the control arms [NRT or ENNDS (no nicotine)] than in the ENDS arm, except for shortness of breath in two studies (highest in the ENNDS arm) and cough in one study (highest in NRT arm) (Supplementary file Appendix 9).
Level and quality of evidence
The authors assigned a Level-2 evidence rating using the Centre for Evidence-Based Medicine levels of evidence guidelines56 as we had eight RCTs in the NMA, but seven of the eight trials had a high risk of bias. With respect to the certainty of evidence57,58, there is low certainty of evidence that e-cigarettes or ENDS have the same levels of success in achieving smoking cessation as the regulated gold standard nicotine replacement therapies for cessation25. The low certainty is based on the results of the NMA at 6 months, because of the high-risk of bias in seven of the eight trials, the low number of successful events in the trials, and the high dropout rates.
There is a very low certainty of evidence or inconclusive evidence that ENDS have the same levels of or more success in achieving smoking cessation as other medically approved cessation interventions, based on three trials all with different comparators, for smoking cessation at 52 weeks.
DISCUSSION
The incidences of smoking cessation at 24–26 weeks, derived from this NMA of eight trials, were similar between ENDS and NRT groups (RR=1.17; 95% CrI: 0.65–1.86; low certainty of evidence). Three sensitivity analyses indicate that the main findings for 24–26 weeks were robust to assumptions relating to the smoking history of participants and inclusion of unverified data, and to the assumption of equivalence in nicotine dose. An NMA of smoking cessation at 52 weeks was not undertaken due to very limited data, and a narrative synthesis of evidence for effectiveness of e-cigarettes in smoking cessation at this time showed substantial uncertainly in individual RCT results. No serious adverse events were designated as being related to treatment in the six studies that intended to document serious adverse events; however, the procedure for determining if a serious adverse event was related to the smoking cessation intervention was often unclear. In general, safety and health impacts of e-cigarettes beyond 12 months are not yet established.
The complex and emergent nature of e-cigarettes presents a key challenge for public health policy makers and healthcare professionals, so it is critical that evidence synthesis is independent, transparent, makes best use of available and up-to-date evidence so as to usefully inform critical population and individual level decisions.
Strengths and limitations
There are a number of strengths to this systematic review. First, the systematic review and meta-analysis was conducted by a group who were unaligned to the conduct of the primary studies providing an independent assessment of evidence. Second, a comprehensive, robust and reproducible search strategy was used to capture all relevant trials. Third, different endpoints across studies have been disentangled and reported separated for accuracy. Finally, NMA, augmented with well-reasoned sensitivity analyses based on feasibility and appropriateness assessment, was chosen as the preferred method to fully analyze the available evidence base and optimizes precision; such analyses can deal with multi-arm trials.
A number of limitations in the primary studies restricted the extent of our evidence synthesis and our certainty in the findings. In general, the quality of the primary studies synthesized in this review was low, with all but one study assessed as being at high-risk of bias. Many studies were small, with high loss-to-follow-up and low numbers of participants achieving smoking abstinence, even at the proximal 24–26 weeks endpoint. Significant concerns regarding comparators and quality prevented synthesis of the 52-week endpoint. While loss-to-follow-up is a common limitation in trials of smoking cessation interventions and procedures have been agreed for handling outcomes, this source of information error was different in the included trials, with loss-to-follow-up generally greater in NRT treated trial arms, which underscores the importance of achieving both high and equal response rates across compared groups to limit bias59. This dependence on the quality of primary research is a well-recognized inherent limitation of evidence synthesis, and efforts have been made in this review to address this challenge through transparent reporting60, disentanglement of endpoints, and sensitivity analyses. E-cigarettes are not a standardized intervention. A variety of first-and second-generation e-cigarettes were tested, and the nicotine doses varied, although the impact of dose was found to be robust in sensitivity analysis. NRT dosing in the comparator arm was commonly monotherapy and sometimes includes less intense dosing. While monotherapy and lower dosage may replicate how some people use NRT in a self-managed cessation attempt, it is generally at odds with well-established evidence and clinical practice in treating tobacco addiction where best practice advice on NRT is combination therapy at more intense and more optimal nicotine dosages18. These issues with both the active and comparison arms of the studies present challenges to generalizability.
A limitation of our NMA is that we did not include studies that reported data for NRT versus control treatments alone. The reason for this was two-fold, firstly our primary focus was on the efficacy of e-cigarettes versus standard care for smoking cessation, the efficacy of NRT for this purpose has already been proven61. Secondly, this research team did not have the resources required to expand the search to include data for this comparison, it would have at least tripled the workload and meant that the research team would not have been able to address the primary research question. Future research could consider an expanded network meta-analysis of all smoking cessation treatments, noting that this would be a resource intensive project.
This review concludes that e-cigarettes with nicotine may be as effective as NRT in achieving smoking cessation at 24–26 weeks (eight RCTs). Previous reviews in this important area have been mixed. Rahman et al.23 pooled data from two trials and found that ENDS, compared with ENNDS (no nicotine), helped smokers to stop smoking long-term23. On the other hand, a 2016 review by Kalkhoran and Glatzlost20 concluded that e-cigarettes are associated with significantly lower quit rates among smokers20. More recent reviews by El Dib et al.17 and Khoudigian et al.19, based on two and three trials, respectively, report findings similar to ours – that the incidences of smoking cessation at 24 or 26 weeks for ENDS versus ENNDS (no nicotine) indicate that an e-cigarette with nicotine is marginally better than one without nicotine, but that this result is not statistically significantly and there is a high-level of uncertainty. A systematic review by Malas et al.21 in 2016, elected not to conduct a meta-analysis because of the heterogeneity of the data. They concluded that, while the majority of included studies (mostly non-RCTs) suggested a positive relationship between ENDS and smoking cessation, the evidence remained inconclusive due to the low quality of the published data. According to a 2020 World Health Organization report, based mostly on the US Academies of Sciences systematic review, some types of ENDS aid in smoking cessation in certain circumstances, but the evidence is insufficient to issue a general recommendation to use any type of e-cigarette (nicotine or non-nicotine) as a cessation aid for all smokers62.
Wang et al.24 published a systematic review and meta-analysis in 2021, looking at both observational and RCT data. Observational and RCT data were analyzed separately, with the observational indicating that ENDS were not associated with increased smoking cessation but the RCT analysis found that ENDS were associated with increased smoking cessation in a controlled situation.
A systematic review leading to NMA was also published in 2021 by Chan et al.22 who found that ENDS users were more likely to quit smoking than control or NRT users; however, they stipulated that more high quality studies are required to ascertain the true effect of ENDS on smoking cessation. Chan et al.22 made a number of assumptions in their analysis that were different to ours including: pooling of data over multiple timepoints and pooling of all non-nicotine treatment arms, i.e. assuming non-nicotine e-cigarettes are the same as usual care.
While this manuscript was being completed, a revision of a Cochrane review of e-cigarettes for smoking cessation18, which is now designated as a living review, was published. While there are similarities in the research question, there are a number of differences between this work and that presented in the Cochrane review. The most important of these relate to the type and timing of the outcomes that are summarized. In our current work, to be consistent with standards in the area of smoking cessation trials26, we have excluded studies where the outcome is not continuous abstinence without relapse throughout the follow-up period, e.g. Lee et al.54 which measured 7-day point prevalence abstinence only. In addition, we have separately analyzed outcomes at 24 or 26 weeks and those reported at 52 weeks rather than take data at the longest follow-up only and present these to the systematic review user as the same endpoint. Finally, a key difference is the use of Network Meta-Analysis, which means a broader collection of indirect evidence (when compared to pairwise meta-analysis) is included in the synthesis. We consider our decisions clinically and methodologically sound. The Cochrane review reported that there is moderate-certainty evidence that ENDS increase quit rates compared to NRT. This conclusion is based on a pairwise meta-analysis of three RCTs of which two reported data at 6 months, one at 12 months and one did not conform to the strict definition of smoking abstinence. We report low and very low certainty of evidence in our results because of the high risk of bias in most of the included trials, the high numbers lost to follow-up in the trials, and the low success rates of all trial interventions. The final difference is that the review presented in this article employed the newer Cochrane Collaboration ROB2 tool while Hartman-Boyce et al.18 used the previous version 1 of this tool, which may explain differences in the two groups assessment of bias results.
Future perspectives
To better inform policy and clinical practice, future studies of e-cigarettes should be designed with comparator arms that offer participants intense and optimal NRT as well as other more effective smoking cessation interventions. The effectiveness of e-cigarettes at 24–26 and 52 weeks needs to be established as to date results are mixed and of low or very low quality. The question of whether e-cigarettes may have different effectiveness for different populations of smokers also needs to be addressed, since the trade-offs at individual patient level between the harms, benefits and uncertainties are different. Further long-term large-scale multi-country RCTs are needed to assess the efficacy and safety of electronic cigarettes (e-cigarettes) in helping people who smoke to achieve abstinence. If e-cigarettes are to be used as a treatment for smoking cessation, then they need to have an established long-term safety profile. This links with the questions of their regulation as medical or consumer products, which will be especially important for clinicians. Recommending e-cigarettes has been demonstrated to lead to their continuing use by people who are successful in stopping smoking tobacco cigarettes as well as by people who experience unsuccessful quit attempts and end up using both e-cigarettes and tobacco cigarettes (dual use)45,63. Some of these people may not have tried e-cigarettes in the absence of a therapeutic recommendation45,63. In a mapping exercise on the harms and benefits of e-cigarettes, McCarthy et al.65 noted that many studies showed that dual use of e-cigarettes and conventional tobacco cigarettes was not less harmful than smoking conventional tobacco cigarettes only, thereby raising questions about the smoking reduction benefit of e-cigarettes. Any future studies on e-cigarettes and smoking cessation need to monitor on-going use of e-cigarettes, in particular dual use, as a consequence of recommending e-cigarette use for smoking cessation. While reporting a finding of greater effectiveness of e-cigarettes compared with NRT with moderate certainty, the recent Cochrane review also identifies a need for more studies with higher quality to build a more reliable evidence-base.
The systematic review evidence on the efficacy of ENDS for smoking cessation to date shows contradictory results. There are, of course, explanations for these differences, however, it is abundantly clear that if research groups can use standard evidence synthesis techniques and generate different results, then further high-quality primary evidence is urgently required.
The scale of disease, disability and premature mortality caused by smoking continues to demand an urgent response64, which not only includes stronger initiation prevention, but which also provides more effective cessation support to help save the lives of people who currently smoke. Policy-makers and healthcare professionals alike are struggling to navigate and resolve the often divisive debate65 on what, if any, role e-cigarettes may play in this context6-8. Both sets of decision-makers face trade-offs between harms, benefits and uncertainties at the individual level, however, policy-makers face the added consideration of potential trade-offs across population groups and between short- and long-term goals for public health.
CONCLUSIONS
Much needed progress is being made towards providing robust and precise scientific evidence to enable clinical and policy decisions about e-cigarettes to be made with greater confidence12. However, the fact that this review and recent Cochrane review have arrived at different conclusions, largely through differences in assumptions around the data included and the overall method of analysis, while both following accepted evidence-synthesis practice, will add to concerns about the role of systematic reviews in supporting decision-making66,67. This should not be the case, however. Both reviews are aligned on the need for further well-designed, adequately powered, and carefully conducted primary studies with clearly reported cessation and safety outcomes to support clinical and policy decision-making. Overall, these reviews indicate that the research question ‘should ENDS be recommended to aid smoking cessation?’ is far from answered. Further research should be synthesized by review teams that are independent of the primary trial groups to avoid investigator bias to increase transparency and trust in findings.
However, urgent action to tackle the harms of smoking based on what we know is safe and works cannot and should not await resolving questions about e-cigarettes. Healthcare professionals must work to close the implementation gap between existing knowledge on smoking cessation interventions with well-established effectiveness and safety profiles and the reality of care, as it is too often experienced by people who smoke who commonly miss the opportunity to receive clinical advice and support at a time when it could have greatest impact68-70. Policy-makers should act now to ensure that they have fully protected children and young people from the harms of e-cigarettes and the potential to undermine progress in tobacco control through facilitating smoking initiation71. While determining whether the risk-benefit profile of e-cigarettes in some population groups is best mobilized through their regulation as consumer products or through the same regulation as other licensed medicines72, there is much more policy-makers can do to immediately support healthcare professionals and patients to maximize the uptake of existing, well-regulated smoking cessation interventions73-75.
The e-cigarette debate continues to predominate tobacco control internationally. Do we need to await a final verdict on a single potential game-changer to take action now to change the tobacco control game and bring it to an end76? While our study indicates that research evidence is not yet sufficient in volume or quality to conclude the e-cigarette debate, it affirms that clinician and policy-makers urgency to tackle the harms of smoking can be focused with confidence on augmenting support for interventions with well-proven safety and effectiveness.