INTRODUCTION
During the last two decades, electronic cigarettes (e-cigarettes) have been the subject of a heated debate and polarized responses within the field of tobacco control and prevention1-3. On the one hand, it has been suggested that e-cigarettes may present a less harmful alternative to combustible cigarettes that potentially could lead to beneficial effects on individual and public health4,5. On the other hand, e-cigarette use is associated with increased risk for smoking initiation among non-smokers6. In addition, there is no conclusive evidence that e-cigarette use facilitates smoking cessation7 or contributes to harm reduction8. The results of systematic reviews of the association between e-cigarette use and smoking cessation have shown inconsistent results. Two of the earlier systematic reviews published between 2014 and 2016, concluded that there was an association between e-cigarette use and smoking cessation9,10, while another review found that e-cigarette use reduced the likelihood of smoking cessation11. However, these reviews included few studies and thus based the conclusions on small samples. More recent systematic reviews have concluded that it was not possible to determine whether there was an association between e-cigarette use and subsequent smoking cessation12,13. The publication rate of studies about e-cigarette use and smoking cessation is rapidly growing and in the most recently updated systematic review from the Cochrane Library, the evidence level was rated as moderate7 compared to low in the previous editions9,14. However, some of these reviews include publications with study designs that are subject to numerous limitations, such as studies with a cross-sectional design or present pooled data from cohort studies and randomized controlled trials (RCT) in the same analysis. The distinction between observational and experimental studies is crucial in this domain, because of the potential of bias by confounding in the former and because of the likely selection of motivated smokers in the latter. The aim of this systematic review and meta-analysis was to assess the association between e-cigarette use and subsequent smoking cessation in cohort studies and RCTs, respectively.
This review is based on data previously published as a report from The Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) (Report 312, SBU 2020/431)15. A summary of the protocol was published in Swedish at the SBU website in February 2019 (https://www.sbu.se/sv/publikationer/forstudie-sammanfattning/forstudie-tobaksprevention/) and the complete protocol can be requested (from registrator@sbu.se).
METHODS
Research question
This review had the following research question: ‘Is there evidence for an association between e-cigarette use and subsequent smoking cessation among people smoking tobacco in the beginning of the study period and do the results differ between cohort studies and RCTs?’. The PICO (Population, Intervention, Comparison, and Outcome) frame used to establish the eligibility criteria is presented in Tables 1 and 2.
Table 1
PICO frame in cohort studies
Table 2
PICO frame in randomized controlled trials
Literature search
A comprehensive literature search of EMBASE, Cochrane library, Scopus, Pubmed Health, NICE evidence search, PROSPERO, CRD (DARE), PsycInfo and PubMed including Medline was conducted in December 2018 and updated 11 November 2019. Search terms were based on the following keywords: [electronic cigarettes, vaping, vaporized nicotine, electronic nicotine delivery system] combined with [cigarettes, smoking, combustible tobacco]. We also asked researchers in the field to provide lists of relevant articles and we read reports from Swedish authorities and Health Technology Assessment organizations for background material. Details on the literature search can be found in Supplementary file Table S1.
Inclusion and exclusion criteria
Inclusion criteria were: published in peer review journals between 1 January 1990 and 11 November 2019; longitudinal design or randomized controlled trial, with at least one baseline and one follow-up measure; included a parallel control group; at least 3 months follow-up; presentation of the results in a way that could allow the calculations of risk ratios or equivalent association measures (e.g. odds ratios); and written in English, Swedish, Norwegian or Danish. Exclusion criteria were: conference abstracts, book chapters or articles not reporting empirical results; and studies based on selected samples and not representative of the underlying general populations (e.g. military personnel, patients with specific diagnoses, including psychiatric disorders). Studies reporting conflicts of interest were not excluded, but information on conflicts of interest presented in the included articles is presented in Supplementary file Table S2. The study selection processes, and the articles excluded in each step are further outlined in Figure 1. Details on excluded articles can be found in Supplementary file Tables S3 and S4.
Assessment of risk of bias
The literature was assessed using a priori established protocols, and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; http://www.prisma-statement.org/). The screening of the publications was performed using established protocols based on Cochrane’s templates: ROBINS-I and ROBINS-E for the cohort studies and the SBU risk-of-bias tool for randomized trials based on the Cochrane RoB 2 template for the RCTs (https://www.riskofbias.info/). These templates have been developed by building upon tools for risk-of-bias assessment of randomized trials, diagnostic test accuracy studies and observational studies of interventions, and has been developed by members of the Cochrane Bias Methods Group and the Cochrane Non-Randomized Studies of Interventions Methods Group16. The title and abstract of each publication were initially reviewed by two of the authors. Disagreements were resolved by a third reviewer. None of the reviewers had any conflicts of interest regarding the rated publications.
Publications read in full text but rated as not eligible for this review are listed in Supplementary file Table S3. Those considered eligible underwent a risk-of-bias assessment independently by two of the authors. Only prospective studies with a low or moderate risk-of-bias in all domains were included in the analysis (Figure 1). For observational studies, the categories were: selection, exposure, judgement, drop-out rate, or reporting. For RCTs, the categories were: randomization, deviation from planned intervention, attrition, measurement of outcome, or reporting. Articles excluded due to high risk-of-bias are presented in Supplementary file Table S4.
Data extraction and statistical analysis
Main features extracted from the selected studies were: authors, date of publication, study design, subjects and sample size, and main quantitative results (Supplementary file Tables S5 and S6). We included studies where it was possible to retrieve either absolute numbers or percentages for each group (unadjusted analysis), or where the study presented the results as both adjusted and unadjusted odds ratios. All analyses were conducted according to the a priori analysis plan, with two main outcomes: non-use of combustible tobacco and non-use for at least 30 days at the time of the follow-up. Unadjusted and adjusted data were analyzed separately, and studies where odds ratios could not be calculated were not included in the meta-analyses but contributed to the narrative summary.
To assess the association between e-cigarette-use and subsequent smoking cessation, relevant data were combined in a random-effects meta-analysis, combined with inverse variance using Review Manager 5.3 [Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014]. We also considered the following subgroups: duration of follow-up (≤6 months or >6 months), age (<18 years or ≥18 years, based on the legal age for purchasing tobacco products in many countries) and sex (male or female). We also conducted separate analyses for cohort and RCT studies. Subgroups for further analysis were defined a priori. During the evaluation, several studies were shown to build on the same or overlapping cohorts. In these cases, only the most complete data set from the overlapping study populations was used in our meta-analysis. If a study presented several sets of adjusted data, with different numbers of confounders, the least adjusted model that contained the variables sex, age and socioeconomic factors was chosen. This was done to make the estimates included in the analysis as comparable as possible. Some observational studies measured the exposure (e-cigarette use) at baseline, but some studies measured the exposure at follow-up and the meta-analyses are presented separately for these two different methods. A putative publication bias was assessed using a funnel plot.
Grading of evidence
We followed the GRADE guidelines to appraise the strength of evidence for the presence of the association, resulting from the meta-analysis17-20. In this approach, direct evidence from longitudinal studies starts at high certainty (⊕⊕⊕⊕) and is downgraded based on risk-of-bias, indirectness, imprecision, and inconsistency to levels of moderate (⊕⊕⊕), low (⊕⊕) and very low (⊕) certainty of evidence. Both unadjusted and adjusted estimates were taken into account when grading the evidence. Grading was performed separately for observational studies and RCTs.
RESULTS
Description of the publications included in the review
In total, 28 peer-reviewed publications based on 26 cohort studies, and 8 publications from 7 RCTs were included. Of the RCT studies, data from one of the trials are presented in two of the publications, and one publication is included narratively.
Cohort studies
The included studies are described in detail in the Supplementary file Table S5. Twenty of the cohort studies were performed in the USA21-42, one in Canada43, and five in Europe44-48. The study populations included on average 53% females, varying from 43%34 to 100%23. Three of the cohorts included individuals <18 years21,30,37,43, two included a population with a mixed age span33,48, while all other articles included adults aged ≥18 years at baseline.
The educational level of participants >18 years was reported in 13 of the studies24-26,28,29,32-35,38-40,42. On average, 60% of the participants in these studies had attended school for 12 years or longer. The educational level varied from 11% with at least college level40 to 88% with at least four years at college24. The other studies did not report educational level. Three studies collected data through questionnaires distributed during school hours21,30,37,43, five studies used questionnaires distributed by mail or in other ways29,33,35,39,46, eleven studies collected data through in-person interviews23,26,28,31,32,34,36,40,45,47, and the others used web-based surveys24,25,27,38,42,48. The follow-up time varied from 6 months to 4 years.
Most studies defined smoking at baseline based on smoking during the past 30 days, while eight studies had other definitions of tobacco smoking, for instance ever smokers, established smokers, tobacco smokers, or as having smoked at least 100 cigarettes in their lifetime and now smoking at least on some days21,24,27,29,33,34,39,48. In two of the studies, the participants had plans to quit smoking35,40, in one study 28–50% of the participants intended to quit smoking29, and in one study the participants reported no intention to quit in the next 30 days28. The other studies presented no information on the participants’ intention or motivation to quit smoking.
The exposure group is described as e-cigarette-users in most studies23,24,26,28,31,32,34-36,41-48, as current e-cigarette users in seven studies24,28,29,42,43,46,48, and as ever e-cigarette users in two studies30,39. In two studies, the exposure group is referred to as ENDS users (electronic nicotine delivery systems), including all types of electronic vapor products27,33. The control group is referred to as never e-cigarette users21,25,27,29,30,38,39, non-users of e-cigarettes23,24,26,28,31,32,34-36,41-48, never ENDS users33, or users of nicotine replacement therapy (NRT), varenicline, buproprion, or no aid used40.
Randomized controlled trials
The included studies are described in detail in the Supplementary file Table S6. Of the RCTs, two were performed in the USA49,50, three in Europe51-53, two in New Zealand54-56, and one in Korea57. On average, the trials included 42% women, with a range between 0%57 to 66%56. All RCTs included participants aged >18 years at baseline. One study reported that 25% of the participants had college education or higher50, one reported that 64% of the participants had attended school for at least 12 years56, while the other studies did not report educational level. All studies collected data through in-person interviews. The follow-up time varied between 3 and 12 months.
Five studies defined tobacco smoking at baseline based on smoking during the past 30 days49,52-55,57 while the other studies employed other definitions such as current cigarette smokers of more than two cigarettes per day, having smoked at least once in the last seven days50, adult smokers51, or tobacco smokers56. The participants in the RCTs wanted to quit smoking54-57, were motivated to quit smoking53, were recruited from smoking cessation programs51, were worried about the health effects of smoking and were willing to try new alternatives49, or were waiting for surgery50. In only one study, wanting to quit smoking was not an inclusion criterion52.
The exposure group was described as assigned to e-cigarette use in all studies, and the participants received e-cigarettes free-of-charge. In three trials, presented in four publications, participants were assigned either e-cigarettes with or without nicotine, but in the present analyses these groups were combined53-56. The other studies included e-cigarettes with nicotine only49-52,57. The control groups included participants not receiving e-cigarettes free-of-charge49,52, participants with access to nicotine patches or gum free-of-charge50,54-57, or participants offered telephone counseling to quit smoking53.
Smoking cessation among e-cigarette users and non-users in cohort studies
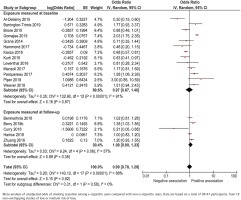
Unadjusted data were presented in 18 of the included studies, including a total of 39147 participants (Figure 2). Among baseline smokers, the unadjusted odds ratio for smoking cessation ranged between 0.30 and 3.00 across studies that measured e-cigarette use at baseline, and 0.21 to 1.38 across studies measuring e-cigarette use retrospectively at follow-up. When combining the data in a random-effects meta-analysis, the pooled unadjusted odds ratio for smoking cessation was 0.97 (95% CI: 0.67–1.40) and 1.09 (95% CI: 0.90–1.33), respectively (Figure 2).
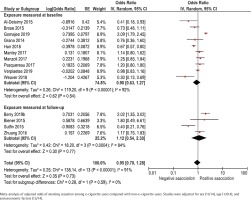
Adjusted results were presented in 14 studies (Figure 3). Adjustments were made for sex (11/14), age (13/14) and socioeconomic factors (13/14), but there was a large heterogeneity with respect to how these adjustments were made. The adjusted odds ratio for smoking cessation ranged between 0.30 and 2.09 for studies that measured e-cigarette use at baseline, and between 0.40 and 2.02 for studies that measured e-cigarette use retrospectively at follow-up. The pooled adjusted odds ratio for smoking cessation was 0.90 (95% CI: 0.63–1.27) for studies measuring e-cigarette use at baseline and 1.12 (95% CI: 0.54–2.30) for studies measuring e-cigarette use retrospectively at follow-up (Figure 3).
Stratified analyses of studies with a short (≤6 months) or a long (>6 months) follow-up time (Supplementary file Figure S1), studies among adolescents (<18 years) or adults (≥18 years) (Supplementary file Figure S2), and one study stratified by sex (Supplementary file Figure S3) showed similar results.
Certainty of evidence – cohort studies
The strength of the evidence concerning the association between e-cigarette use and smoking cessation was deemed to be very low (⊕) due to risk of bias in any of the categories: selection, exposure, judgement or reporting, inconsistency or imprecision between studies, or that the material was based on few studies or few participants (Table 3). For both the unadjusted and adjusted analyses, the direction of the association was inconsistent.
Table 3
E-cigarette use and subsequent smoking cessation in cohort studies
| Association with smoking cessation | Participants | Number of studies (adjusted) | Risk difference | Odds ratio | Certainty of evidence | Downgrading |
|---|---|---|---|---|---|---|
| RD (95% CI)* | OR (95% CI) | |||||
| Full material | 39147 | 18 (14) | -0.01 (-0.03–0.02) | OR 0.99 (0.78–1.33) AOR 0.95 (0.70–1.28) | Very low (⊕) | -1 risk of biasa -1 inconsistencyb,c -1 imprecisiond,e |
| Short follow-up (≤6 months) | 3474 | 3 (4) | 0.03 (0.00–0.05) | OR 1.49 (1.04–2.13) AOR 0.99 (0.69–1.44) | Very low (⊕) | -1 risk of biasa -1 inconsistencyb -1 imprecisione |
| Long follow-up (>6 months) | 36029 | 16 (12) | -0.01 (-0.03–0.02) | OR 0.96 (0.74–1.24) AOR 0.96 (0.70–1.33) | Very low (⊕) | -1 risk of biasa -1 inconsistencyb,c -1 imprecisiond,e |
| <18 years | 331 | 3 (0) | -0.12 (-0.25–0.01) | OR 0.93 (0.40–2.14) AOR Not available | Very low (⊕) | -2 risk of biasa,f -1 material with several limitationsb,d,g,h |
| ≥18 years | 35275 | 14 (12) | -0.01 (-0.03–0.01) | OR 0.96 (0.73–1.25) AOR 0.96 (0.70–1.33) | Very low (⊕) | -1 risk of biasa -1 inconsistencyb,c -1 imprecisiond,e |
| Women | 0 | 0 (1) | Not available | OR Not available AOR 0.94 (0.74–1.19) | Very low (⊕) | -1 risk of biasa -1 imprecisione -1 material with several limitationsf,g |
| Men | 0 | 0 (1) | Not available | OR Not available AOR 1.00 (0.79–1.27) | Very low (⊕) | -1 risk of biasa -1 imprecisione -1 material with several limitationsf,g |
b The confidence intervals of individual studies include 1.0, indicating no statistically significant association.
d The pooled estimate for the unadjusted results includes 1.0, indicating no statistically significant association.
Smoking cessation among e-cigarette users and non-users in RCTs
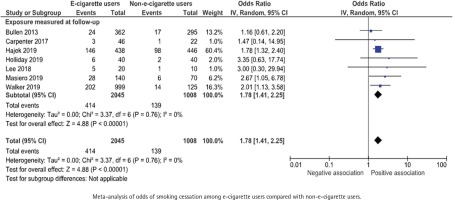
Number or percentage of participants that had quit smoking was presented in all 8 studies, including a total of 3203 participants. One study was included narratively as the outcome was presented as a continuous variable57. All included RCTs showed a positive association between e-cigarette use and smoking cessation with odds ratios for smoking cessation ranging between 1.16 and 3.35 (Figure 4). When combining the data in a random-effects meta-analysis, the pooled odds ratio for smoking cessation was 1.78 (95% CI: 1.41–2.25) for e-cigarette users compared with non-users (Figure 4).
Stratified analyses of studies with a short (≤6 months) or a long (>6 months) follow-up time (Supplementary file Figure S4), and studies among adults (aged ≥18 years) (Supplementary file Figure S5) showed similar results. Studies stratified by sex or that included adolescents (aged <18 years) were not available.
Certainty of evidence – RCTs
The strength of the evidence concerning the association between e-cigarette use and smoking cessation was deemed to be low (⊕⊕) due to risk of bias in any of the categories: randomization, deviation from planned intervention, attrition, measurement of outcome or reporting. Other reasons for downgrading were inconsistency between studies, mainly regarding differences in control alternatives, or that there was a lack of blinding, short follow-up time, limited number of studies, or small sample size (Table 4).
Table 4
E-cigarette use and subsequent smoking cessation in randomized controlled trials
| Association with smoking cessation | Participants | Number of studies | Risk difference | Odds ratio | Certainty of evidence | Down rating |
|---|---|---|---|---|---|---|
| RD (95% CI)* | OR (95% CI) | |||||
| Full material | 3203 | 8** | 0.07 (0.03–0.12) | 1.78 (1.41–2.25) | Low (⊕⊕) | -1 risk of biasa -1 inconsistencyb |
| Short follow-up (≤6 months) | 3203 | 8** | 0.06 (0.02–0.10) | 1.67 (1.32–2.11) | Low (⊕⊕) | -1 risk of biasa -1 inconsistencyb |
| Long follow-up (>6 months) | 884 | 1 | 0.09 (0.04–0.14) | 2.00 (1.38–2.89) | Low (⊕⊕) | -1 risk of biasa -1 material with several limitationsc,d |
| <18 years | Not available | |||||
| ≥18 years | 3203 | 8** | 0.07 (0.03–0.12) | 1.78 (1.41–2.25) | Low (⊕⊕) | -1 risk of biasa -1 inconsistencyb |
| Women | Not available | |||||
| Men | 150 | 1** | Not available | Not available | Low (⊕⊕) | -1 risk of biasa -1 material with several limitationsc,d |
** One study was included narratively. Outcome: 7-day point prevalence of abstinence after 24 weeks. Among participants allocated nicotine gum 29.3% were abstinent, and 22.7% among those allocated e-cigarettes. No statistically significant difference between groups.
Smoking cessation for at least 30 days among e-cigarette users and non-users
For the more strict outcome of self-reported smoking cessation for at least 30 days, 15 cohort studies presented in 17 articles21,22,25,27,29-33,37-41,47,48, and four RCTs were included51,53,56,57, of which one is presented narratively57.
Cohort studies
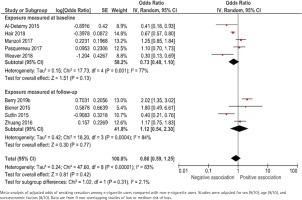
Nine studies, with a total of 13588 participants, present unadjusted data (Figure 5). The odds ratios ranged between 0.30 and 1.51 for studies that measured e-cigarette use at baseline, and 1.02 to 1.20 for studies that measured e-cigarette use retrospectively at follow-up. The pooled unadjusted odds ratio for smoking cessation for at least 30 days was 0.76 (95% CI: 0.45–1.26) and 1.05 (95% CI: 0.93–1.19), respectively. Adjusted data were presented in 10 studies. The data were adjusted for sex (9/10), age (9/10) and socioeconomic factors (8/10), but there was a large heterogeneity with respect to how these adjustments were made. The pooled adjusted odds ratio was 0.73 (95% CI: 0.48–1.10) and 1.12 (95% CI: 0.54–2.30), respectively (Figure 6).
Figure 5
E-cigarette use and subsequent smoking cessation for at least 30 days in cohort studies, unadjusted analyses
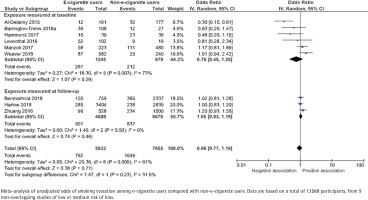
Figure 6
E-cigarette use and smoking cessation for at least 30 days in cohort studies, adjusted analyses
Stratified analysis of studies with a short (≤6 months) or a long (>6 months) follow-up time (Supplementary file Figure S6), and studies among adolescents (aged <18 years) or adults (aged ≥18 years) showed a similar result (Supplementary file Figure S7). Stratified data by sex or adjusted analyses among adolescents were not available.
Certainty of evidence – cohort studies
The strength of the evidence concerning the association between e-cigarette use and smoking cessation for at least 30 days in cohort studies was deemed to be very low (⊕) due to risk of bias in any of the categories: selection, exposure, judgement or reporting, heterogeneity or imprecision between studies, or that the material was based on few studies or few participants (Supplementary file Table S7).
Randomized controlled trials
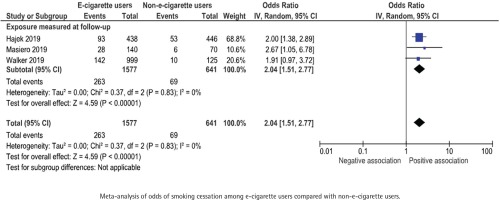
Three studies, with a total of 2218 participants were included in the meta-analysis. One study was included narratively, as it presents the proportion of participants that achieved smoking cessation lasting from the 9th until the 24th week of the study57. All included RCTs showed a positive association between e-cigarette use and smoking cessation for at least 30 days and the odds ratios ranged between 1.91 to 2.67 for e-cigarette users compared with non-users. The pooled odds ratio was 2.04 (95% CI: 1.51–2.77) (Figure 7).
Figure 7
E-cigarette use and subsequent smoking cessation for at least 30 days in randomized controlled trials
Stratified analysis of studies with a short (≤6 months) or a long (>6 months) follow-up time (Supplementary file Figure S8), and studies among adults (aged ≥18 years) showed a similar result (Supplementary file Figure S9). Studies presenting data of adolescents (aged <18 years) or women, were not available. One study was performed only among men, and was included narratively (Supplementary file Table S7).
Certainty of evidence – RCTs
The strength of the evidence concerning the association between e-cigarette use and smoking cessation for at least 30 days in RCTs was deemed to be low (⊕⊕) due to risk of bias in any of the categories: randomization, deviation from planned intervention, attrition, measurement of outcome, or reporting. Other reasons for downgrading were a limited number of studies and small sample size (Supplementary file Table S7).
Assessment of publication bias
Funnel plots did not indicate any publication bias in the material (Supplemental file Figure S10 A-C).
We did not find quality evidence for an association between e-cigarette use and smoking cessation. Although RCTs tended to support a more positive association between e-cigarette use and smoking cessation than the cohort studies, the grading of evidence was consistently low.
DISCUSSION
In this systematic review and meta-analysis, including both observational studies with a longitudinal design and experimental studies, we did not find quality evidence for an association between e-cigarette use and smoking cessation. However, the direction of the association differed between observational and experimental studies. In fact, while the pooled estimates from observational studies were close to the null, those from RCTs indicated in all cases that the use of e-cigarettes compared to control situations was associated with higher likelihood of smoking cessation (70–80% in the pooled results). In one of the few available systematic reviews that distinguished between observational studies and RCTs, the authors concluded that e-cigarette use was associated with smoking cessation in RCTs but was not associated with smoking cessation in the general adult population58. Based on these findings, the authors suggest that e-cigarettes should not be approved as a consumer product, but they could be considered as a prescription therapy for smoking cessation.
The different results between observational studies and RCTs can be explained by several features. First, controlling for confounders is stricter in RCTs than in observational studies. Other than sex, age and socioeconomic factors, there are many other confounders that may affect the association between e-cigarette use and smoking cessation in observational studies. There are a wide range of reasons for initiating e-cigarette use, for instance to quit smoking, curiosity, the different flavors, lower price than tobacco cigarettes, or due to smoking bans59. Moreover, smoking cessation is associated with several physiological, psychological and social factors such as strength of nicotine dependence, withdrawal symptoms, fear of failure, readiness to make a quit attempt, lack of support, and being around other smokers60. These factors are rarely taken into account in observational studies and may contribute to the different associations with the outcome. For RCTs on the other hand, the vast majority of the studies only included smokers that were motivated to quit. Thus, if the smokers were equally randomized into intervention and control group, the motivation to quit would be the same between the groups and any difference in smoking cessation would be more strongly related to e-cigarette use. However, being motivated to quit could increase the likelihood of a successful outcome enhanced by the use of e-cigarettes and may not be generalizable to smokers in the general population. Nevertheless, in line with a systematic literature review by the Cochrane Library7, we rated the evidence of an association between e-cigarette use and smoking cessation in the RCTs as low, mainly due to limitations in the study design, sample size or choice of control conditions.
Another explanation may be that misclassification of exposure, in this case e-cigarette use, is more common in observational studies. Among those defined as e-cigarette users in the observational studies, we can assume a large variation for instance in the type of e-cigarette used, composition of the e-liquid, level of nicotine, e-cigarette use behavior, and how long they have been an e-cigarette user. On the other hand, the assessment of these features of e-cigarette use and nicotine exposure is complicated by the fact that many e-cigarette users are unaware of the content in nicotine in the e-cigarettes they are using, and it has been shown that the level of nicotine is sometimes incorrectly labelled on the product61. Moreover, there is no standardized method of reporting the level of e-cigarette exposure, at odds with number of cigarettes or pack-years for tobacco cigarette smoking. Therefore, the distinction between different e-cigarette uses or nicotine exposure in relation to smoking cessation is seldom possible.
Although most cohort studies measured e-cigarette use at baseline, some obtained that information retrospectively at the follow-up survey. In the latter studies, the sequential order of e-cigarette use and smoking cessation cannot be fully assessed. It is possible that the participants quit smoking first and then initiated e-cigarette use. Therefore, we chose to present the analyses separately for these two groups of studies. However, regardless of when the exposure was measured, the analyses showed similar results, not supporting the presence of an association between e-cigarette use and smoking cessation in population studies.
While RCTs provide higher level of evidence and are more reliable in the exploration of a causal link, observational studies are more informative on the potential impact of e-cigarettes at a population level. Taken together, we conclude that although e-cigarettes may favor smoking cessation over and above a number of conditions among motivated smokers, their impact at the population level, if any, would likely be small.
Strengths and limitations
The major strength of this review is that it distinguishes between cohort studies and RCTs and bases the conclusion on studies using a longitudinal prospective design. Furthermore, to avoid inclusion of articles that were subject to major limitations our review only included prospective studies with a low or moderate risk of bias and duration of follow-up of at least three months. Limitations were the considerable heterogeneity between studies and measures of e-cigarette use. Most studies did not assess the type of device used, how often the e-cigarette was used, the composition of e-liquid, or whether e-cigarettes contained nicotine or not. The adjustment for potential confounding factors was very different across studies. It was thus not possible to grade the effect size of the association. Grading of evidence thereby relates to the certainty of evidence for the association between e-cigarette use and smoking cessation. The drop-out rate was relatively high in most studies, therefore limiting the statistical power and increasing the potential for selection bias. In this review, we did not exclude articles due to their attrition, but this aspect was considered in the judgment of the risk of bias and in the grading of evidence.
CONCLUSIONS
This review and meta-analysis could not find quality evidence for an association between e-cigarette use and smoking cessation. Although RCTs tended to support a more positive association between e-cigarette use and smoking cessation than the cohort studies, the grading of evidence was consistently low.