INTRODUCTION
Tobacco smoking remains one of the leading causes of preventable morbidity and mortality worldwide, accounting for 8 million deaths per year1,2. Over the last decades, novel nicotine and tobacco containing products such as electronic cigarettes (ECs) and heated tobacco products (HTPs) have been introduced to the market and marketed as safer options for smokers unable to quit conventional cigarettes (CCs)3,4. Understanding the patterns of use of these products is crucial for public health, as the combined use of CCs and novel products could exacerbate health risks by exposing users to multiple sources of harm. Therefore, assessment of the short- and long-term health effects of these products is essential to inform evidence-based regulatory decisions and public health strategies.
Contrary to their intended role as cessation tools for adult smokers, novel tobacco and nicotine products have gained considerable popularity among younger populations, including non-smokers, who initiate nicotine dependence by using these products5-7. Moreover, studies consistently show that a significant proportion of users are dual users, combining these products with conventional cigarettes5,8-10. Evidence from many independent longitudinal studies suggests that these products are not effective in smoking cessation and that dual users are more likely to continue smoking CCs7,11,12. The health risks associated with smoking are well documented and include a wide range of serious conditions, from cardiovascular diseases to cancer. For novel tobacco products, the World Health Organization (WHO), supported by several independent studies, has highlighted the potential risks associated with exposure to harmful substances present in aerosols and emissions1,13-16.
Detailed analyses of patterns of use of ECs and HTPs – exclusively and in combination with CCs – are needed to assess in detail how users approach these products and, in particular, to understand how short-term effects such as adverse events vary with different patterns of product use (e.g. exclusive, dual or poly use). In addition, longitudinal data on the medium- and long-term effects of exclusive and combined use of these products remain scarce, with the first observational studies showing an increased risk of lung cancer and other diseases in EC users and dual users16,17. Such data are essential to assess their long-term effects and public health implications.
In this study, we recruited a large sample of Italian adults, including both smokers and non-smokers, to assess the patterns of use of tobacco and nicotine products and the adverse events associated with their consumption. The present analysis represents the baseline of a longitudinal study that will provide the opportunity to investigate not only the short-term adverse effects associated with the use of these products, but also the development of medium- and long-term health outcomes.
METHODS
The present study is based on cross-sectional data collected at baseline from a longitudinal study of Italian adults. Between October 2023 and March 2024, a sample of adults aged 18–93 years was recruited via Computer-Assisted Web Interviewing (CAWI) from the DOXA (a market research company) online panel. The study sample was based on a non-probability, convenience sampling approach. The study protocol was approved by the Ethics Committee of Mario Negri Institute (Ethics Committee of Fondazione IRCCS Istituto Neurologico Carlo Besta, ID: 09, date: 9 November 2022). The inclusion criteria were an age of at least 18 years and residence in Italy. This baseline sample serves as the foundation for a prospective cohort study that will follow participants over the coming years, allowing future analyses of trends in tobacco and nicotine product use and related health outcomes.
Baseline data collection included information on tobacco and nicotine product consumption (full questions in Supplementary file Box 1), covering patterns of use for each product (CCs, ECs and HTPs), as well as the combination of these products. Patterns of use were categorized into exclusive use (CC-only, EC-only or HTP-only), dual use (combined use of two products), and poly use (combined use of the three products). Individuals included in the study were characterized according to their consumption of CCs, ECs, and HTPs. Participants were asked to report any adverse effects they experienced during or after using each specific device. For each product, users (i.e. current smokers for CCs, and both occasional and regular current users for ECs and HTPs) were asked whether they experienced one or more of a structured list of a priori defined adverse events that could be associated with the use of the product, either during or after its use18: dry cough, sore throat, mouth dryness, vertigo, headache, constriction of the respiratory tract, mouth irritation, nausea, dizziness, burns to the lips, mouth ulcers, and other adverse effects. This approach, based on symptom-specific questions rather than open-ended reporting, was designed to improve data consistency and comparability. The main outcomes considered in the analyses were the reporting of at least one adverse event and the mean number of different adverse events reported.
Statistical analysis
Descriptive analyses, using frequencies and percentages for categorical variables, and means and standard deviations (SD) for continuous variables, were conducted to characterize our sample. The sociodemographic variables included sex (male, female), age (18–24, 25–44, 45–64, >65 years), marital status (married/cohabiting, divorced/separated, widowed, single), economic status (above the Italian mean, average, below the Italian mean), and education level (middle school or lower, high school, university degree or higher), and behavioral variables on tobacco and nicotine products consumption. The frequencies of exclusive and combined use of CCs, ECs and HTPs were assessed to determine patterns of product consumption, including dual and poly use. The frequencies of adverse events experienced during or after product use, were assessed by consumption type (exclusive use, dual use, or poly use) to highlight whether different patterns of use influenced the number of different adverse events reported. Since the number of adverse events was not normally distributed, a negative binomial model was performed to compare the mean number of different adverse events associated with CC use between exclusive CC smokers and CC smokers who also used ECs or HTPs. A multivariable logistic regression model adjusted for possible confounders such as age, sex and smoking intensity (i.e. number of CCs smoked per day) was used to calculate adjusted odds ratios (AORs) of experiencing at least one type of adverse event according to use patterns, comparing exclusive CC smokers with dual users of CCs plus ECs and/or HTPs. These analyses aimed to identify significant differences in adverse event reporting based on usage patterns. All statistical analyses were performed using SAS (version 9.4).
RESULTS
Our sample included 22428 Italian adults (9792 men and 12636 women) with a mean age of 47.9 years (Supplementary file Table 1). Overall, 42.7% of participants reported to be never CC smokers, 24.8% former smokers and 32.5% current smokers. Regarding ECs, 78.6% of participants had never used them, 9.1% were former users and 12.3% were current users, including 7.2% occasional users and 5.1% regular users. Finally, 81.7% of participants reported never using HTPs, 7.8% were former users and 10.6% were current users, including 5.3% occasional and 5.2% regular users (Table 1).
Table 1
Tobacco and nicotine product use among adults included in the study, Italy, 2023–2024 (N=22428)
| Product usea | n (%) |
|---|---|
| Conventional cigarettes | |
| Never | 9581 (42.7) |
| Former | 5554 (24.8) |
| Current | 7293 (32.5) |
| Electronic cigarettes | |
| Never | 17635 (78.6) |
| Past | 2033 (9.1) |
| Current | 2760 (12.3) |
| Occasional | 1616 (7.2) |
| Regular | 1144 (5.1) |
| HTPs | |
| Never | 18313 (81.7) |
| Past | 1746 (7.8) |
| Current | 2,369 (10.6) |
| Occasional | 1195 (5.3) |
| Regular | 1174 (5.2) |
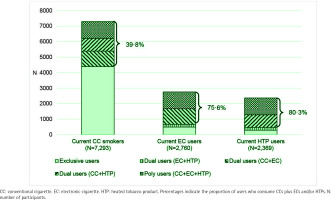
Patterns of exclusive and combined CC, EC and HTP use are detailed in Figure 1 and Supplementary file Table 2. Out of 7293 current smokers of CCs, 60.2% were exclusive smokers of CCs, of 2760 current EC users 17.9% were exclusive users of ECs, and of 2369 current HTP users 12.1% were exclusive users of HTPs. Overall, 82.1% of EC users and 87.9% of HTP users, were dual or poly users. In particular, 75.6% and 80.3% of EC and HTP users, respectively, reported also using CCs. Among CC smokers, the average number of CCs smoked per day was 10.7 and did not significantly differ between exclusive, dual and poly users.
Figure 1
Pattern of exclusive, dual, and poly use among conventional cigarette smokers, electronic cigarette users and heated tobacco product users, Italy, 2023–2024
Participants reported a range of adverse events experienced during or after using each tobacco or nicotine product (Table 2). The mean number of different adverse events reported was 1.41 for CC consumption, 0.78 for EC use and 0.74 for HTP use. Among CC smokers, 31.8% reported no adverse events, 29.0% reported one type of event, 19.8% two, 19.4% reported three or more types of adverse events. Among EC and HTP users 55.3% and 55.5% experienced no adverse effects, respectively, and 8.2% and 6.8%, respectively, reported three or more adverse events. Dry cough was the most frequently reported event for CC smoking (40.0%), followed by sore throat (20.9%), mouth dryness (20.2%) and vertigo (11.9%). For ECs, the most common events were mouth dryness (15.8%), dry cough (15.5%), sore throat (8.9%) and nausea (7.4%). Similarly, HTP users most commonly reported mouth dryness (15.9%), dry cough (15.2%), sore throat (8.2%) and mouth irritation (7.0%) (Table 2; and Supplementary file Figure 1).
Table 2
Number and type of adverse events experienced during or after the use of conventional cigarettes, electronic cigarettes and heated tobacco products among adults included in the study, Italy, 2023–2024 (N=22428)
Among CC smokers, mean number of different adverse events for CC smoking increased from 1.29 (SD=1.36) for exclusive CC smokers to 1.65 (SD=1.61; p<0.001 compared with exclusive CC smokers) for those also using ECs, 1.63 (SD=1.61; p<0.001) for those also using HTP, and 1.73 (SD=1.69; p<0.001) for those using EC and HTP (Figure 2; and Supplementary file Figure 2). In multivariable models adjusted for age, sex and smoking intensity, CC smokers also using EC (AOR=1.32; 95% CI: 1.17–1.50), also using HTP (AOR=1.14; 95% CI: 1.01–1.30) and also using both EC and HTP (AOR=1.50; 95% CI: 1.29–1.75) had a higher likelihood of experiencing at least one adverse event compared to exclusive CC smokers (Supplementary file Table 3)19.
DISCUSSION
In this study, we analyzed baseline data from a large sample of Italian adults to investigate patterns of use of tobacco and nicotine-containing products, as well as adverse events associated with their use. Our findings revealed that exclusive use of ECs and HTPs is uncommon, with most users opting for dual or poly use, combining these products with CCs. Dual users of CCs and novel tobacco and nicotine products reported a higher number of different adverse events during or after cigarette smoking compared with CC-only smokers, highlighting the increasing harm of combining conventional and novel products.
Among current EC users, only fewer than one in five were exclusive users, while the majority combined their use with other tobacco products. Similarly, among current HTP users, only one in eight were exclusive users, while the rest were dual or poly users. Dual use with CCs was particularly prevalent, with three-quarters of EC users and four-fifths of HTP users also smoking CCs. These findings confirm that large majority of users of novel products combine them with existing or acquired smoking behavior, rather than using them as an alternative. This pattern of dual or poly use underlines the limited role of these devices in promoting complete cessation of conventional smoking. Instead, it may reflect a common behavior of using novel devices alongside CCs to obtain nicotine in places where cigarette use is prohibited. Our results are in broad agreement with previous findings. A recent systematic review based on representative samples found that about two-thirds of HTP users were dual users, combining HTP use with conventional cigarettes5. In addition, independent research shows that smokers who use novel products are less likely to quit than exclusive smokers7,11,12. The low prevalence of exclusive use of novel products observed in our sample supports this interpretation and highlights the ineffectiveness of novel tobacco products in promoting effective smoking cessation.
A substantial proportion of CC smokers enrolled in our study reported symptoms such as dry cough, sore throat, and mouth dryness. These results reinforce the extensive body of literature linking smoking to both immediate and long-term health consequences1,2. Interestingly, our data also show that ECs and HTPs are far from free of adverse effects. Users of these devices reported a variety of symptoms, some of which overlap with those experienced by CC users (e.g. dry cough, mouth dryness), and others potentially more specific to these products, such as nausea. These findings are consistent with evidence from the literature and with WHO guidelines, which warn that novel tobacco products emit harmful chemicals that can cause adverse effects1,13-16. While novel products are often marketed as safer alternatives to smoking, our findings highlight their potential to cause immediate discomfort and adverse health effects, raising concerns about their public health impact.
By investigating the relationship between patterns of use and adverse events, we found that with the same intensity of CC smoking, dual users of CCs and novel tobacco products reported a significantly higher number of different adverse events, than exclusive CC smokers. This finding supports a growing hypothesis in the literature that dual use may have worse health consequences than smoking alone. The most recent meta-analysis on the topic found that current dual use of CCs and ECs was associated with 20–40% higher odds of disease, than CC-only smoking16. A recent case-control study in the United States of nearly 5000 lung cancer cases and more than 27000 controls showed that dual users of CCs and ECs had a fourfold increased risk of lung cancer compared with exclusive CC smokers17. This substantial increase in risk among dual users is thought to be due to combined effects of exposure to harmful chemicals in both conventional smoke and aerosol emissions from e-cigarettes19,20. Our findings support this hypothesis by showing that dual users of CCs plus ECs and/or HTPs experience more frequent adverse events, even in the short-term. It is plausible that the combined use of multiple products increases exposure to different toxicants, exacerbates inflammation and may contribute to carcinogenesis and respiratory damage17,21. Further research is needed to confirm these mechanisms and to better understand the long-term effects of dual use, particularly in relation to cancer risk and other chronic diseases.
Limitations
As our sample was recruited using a convenience sampling approach, it is not representative of the general population, limiting the generalizability of the results. However, the distribution of the sample in terms of age, sex and geographical area is comparable to that of the Italian population. In addition, the large sample size allowed us to examine a wide range of patterns of tobacco and nicotine product use with sufficient statistical power to detect meaningful differences. This large sample also allowed detailed analyses of adverse events associated with individual products and their combinations of use, providing insights into the risks of dual and poly use. Another potential limitation is the cross-sectional nature of this baseline analysis, which prevents us from inferring causal relationships between patterns of use and the occurrence of adverse events, as well as determining the temporal sequence of product initiation among dual and poly-users. Therefore, while our findings suggest associations between combined product use and higher likelihood of adverse events, they should not be interpreted as causal. Longitudinal follow-up of the cohort, already planned for the coming years, will address this limitation and allow prospective assessment of adverse events and long-term health effects of these products, which are currently under-researched. Moreover, a potential limitation of this study is that all data were collected through self-reported questionnaires. This approach is inherently subject to potential recall bias, reporting bias, and misclassification in both exposure (product use and intensity) and outcomes (adverse events). Furthermore, residual confounding cannot be ruled out, since we were unable to take into account all the potential covariates (e.g. years of exposure to tobacco/nicotine products and lifestyle factors) that could affect the occurrence of adverse events. Future studies should aim to replicate and expand upon our findings using smaller, more controlled samples with validated measurement tools. Finally, our study focused exclusively on adults, and the small proportion of young adults (aged 18–24 years) in our sample – one in forty – did not allow meaningful stratified analyses. Since adolescents and young adults may show distinct patterns of product use and increased vulnerability to adverse effects, future research should specifically address these age groups.
CONCLUSIONS
Our findings indicate that exclusive use of ECs and HTPs is rare, suggesting that these devices are not being adopted as alternatives to CCs, but that most users combine them with conventional smoking through dual or poly use. These dual users of CCs and novel tobacco and nicotine products have an increased likelihood of experiencing adverse events compared with CC-only smokers. Also, the mean number of different adverse events experienced during or after CC smoking is significantly higher in dual or poly users. To this number, we must add the number of adverse events due to EC or HTP use, which is far from negligible. Therefore, our findings are consistent with the growing evidence of a higher health risk for dual users compared to CC-only smokers, suggesting that ECs and HTPs may undermine rather than support tobacco control efforts. Additional longitudinal and comparative studies are needed to improve our understanding of the health impacts of exclusive and combined use of novel nicotine and tobacco containing products.



