INTRODUCTION
Pediatric practitioners are encouraged to use every healthcare visit as an opportunity to screen parents about tobacco use and counsel parental smokers to quit smoking to improve their child’s health1-4. Although rates of smoking cessation counseling in pediatric primary care settings are increasing, rates of recommending or prescribing pharmacological cessation therapy remain low at 15% and 3%, respectively5. Reasons for low prescription rates by pediatric providers include concerns about lack of knowledge and scope of practice, due to feeling uncomfortable in prescribing medications for parents who are not their patients5-7. Nevertheless, the American Academy of Pediatrics strongly encourages pediatric practitioners to prescribe pharmacological cessation medications in conjunction with counseling during children's healthcare visits to help parents quit smoking2,3.
The provision or prescription and use of Nicotine Replacement Therapy (NRT) is effective in helping adult smokers quit smoking8. NRT helps to increase abstinence by temporarily replacing much of the nicotine obtained from cigarettes1,8-11. NRT has an excellent safety profile with rare cases of associated serious adverse events8. NRT is available over-the-counter and pharmacists are able to prescribe NRT in some states12. NRT is also covered by most insurance plans, including Medicaid. Thus, the use of NRT is strongly encouraged in cessation attempts. NRT has been administered to low-income smokers who present to the adult emergency department (ED) as part of cessation interventions with high rates of acceptance and some success in helping smokers quit9,13-16. To our knowledge, no research studies based on pediatric ED (PED) or pediatric urgent care (UC) settings have examined the direct administration of free NRT to parents as part of a cessation intervention during their child’s emergency visit. To fill this gap, we recently conducted a large randomized controlled trial (RCT) called Healthy Families (NCT02531594), which included cessation counseling and the provision of free NRT to parental smokers during their child’s emergency visit. In this study, our objective was to present the results on the number and characteristics of parents who were interested in free NRT compared to those who were not. For parents interested in NRT, we assessed their eligibility to receive a 6-week supply of free NRT. After 6 weeks, we followed up with parents who received free NRT to assess their uptake, usage patterns, any associated side effects, and interest in receiving another 6-week NRT supply. The results of this study will inform future researchers and practitioners who are planning to administer free NRT to adult smokers in the pediatric emergency setting.
METHODS
Design, participants and IRB approval
We analyzed data from the Healthy Families RCT, a tobacco cessation intervention for parental smokers who presented to the PED or UC of a Midwestern Children’s Hospital from April 2016 through May 2019. Participants in Healthy Families were parents/legal guardians who were current smokers and seeking pediatric care for their child 0-17 years of age. A total of 750 parents were recruited and randomly assigned to either the Screening, Intervention, Referral to Treatment (SBIRT) condition (n=377) or to an attention control condition (n=373). Only data from the 377 parental smokers in the SBIRT condition were analyzed in order to address the study objectives. Healthy Families study details are described elsewhere17, but briefly, parents randomized into the SBIRT condition received face-to-face, tailored smoking cessation counseling that focused on the child’s illness and the option to receive 6 weeks of free NRT (patch or lozenge) if they agreed to set a quit date within 14 days of their child’s emergency visit. Parents were followed up in their homes 6 weeks after the emergency visit and offered an additional 6-week supply of NRT if they reported using at least 80% of the initial 6-week NRT supply and remained interested and eligible to use NRT. The SBIRT interventionists were either clinical research coordinators or social workers who had, at minimum, a college degree and were trained as Tobacco Treatment Specialists at an accredited Tobacco Treatment Specialist Training program (e.g. The ACT Center for Tobacco Treatment, Education and Research).
Results of the cessation outcomes of parents who used NRT in combination with the rest of the SBIRT will be presented elsewhere. Healthy Families was approved by our hospital’s IRB and participant consent was obtained. Since the adult participants were not patients at our hospital, there were concerns regarding dispensing of NRT in this setting. Therefore, we had to seek approval from leadership in clinical research and from the Pharmacy Department to administer free NRT and approval on the criteria used to determine which participants were eligible for NRT. As part of our IRB protocol, we were required to have the SBIRT interventionist call the principal investigator (first author) to review a checklist of contraindications prior to administering NRT. The contraindications were more stringent than recommended18,19 due to initial concerns about NRT administration in our setting. After approximately one year of this practice, our IRB allowed us to amend our protocol and the interventionist only had to call if there were any concerns or questions about responses on the checklist. We were cognizant that combination NRT (e.g. nicotine patches plus lozenges) would likely result in higher long-term quit rates than single NRT for some participants10, but due to the novelty of NRT distribution in our setting for this study, we were allowed to provide only single modality NRT to participants.
Assessments
During the child’s emergency visit, parents completed assessments that included: sociodemographic factors, financial strain (three items, each 1–5 range; items were added and means reported)20, smoking behavior including nicotine dependence and prior quit attempts, readiness to quit smoking as measured by the Contemplation ladder (range: 0–10)21, heavy smoking index (HSI) to assess level of nicotine dependence22, and whether the child was being evaluated in the PED or UC.
After completing the baseline assessments, all parents received smoking cessation counseling, which included information on NRT use17. They were asked if they were interested in NRT, and if so, if they were willing to set a quit date within the next 14 days. All interested parents willing to set a quit date were subsequently asked if they preferred NRT patches or lozenges. They were then screened for eligibility for their first choice of NRT by asking about any potential medical contraindications. If parents were ineligible for their first choice, they were screened for their second choice. Eligible parents with no contraindications who received NRT were told to report any potential NRT-related side effects of concern at any time to study staff. We grouped parents based on their interest in receiving NRT (interested, not interested).
Statistical analysis
Data were collected within a REDCap® database, where data entry branching and limited checking were in place to aid with accuracy and completeness of the information. The majority of the data were electronically entered by the enrolled parent. The data used were exported to SAS® version 9.4 (SAS Institute, Cary, NC) for analysis. Prior to analysis, all data were checked for outliers and consistency. We calculated means and associated standard deviations (SDs), medians with 25th and 75th percentiles to show the interquartile range (IQR), and minimum and maximum values to depict the range. Continuous variables were compared between the ‘interested’ in NRT and ‘not interested’ in NRT groups using Wilcoxon rank-sum test, due to the general skewed nature of the data. Categorical variables were compared between the two NRT interest groups using chi-squared tests. A p-value <0.05 was considered statistically significant.
RESULTS
The majority of parents were female (87.5%), non-Hispanic Black (52.5%), with a mean age (SD) of 33.1 (8.2) years, low income with 61.3% reporting an annual income of <$15000. Parents had a low level of nicotine dependence on average (HSI <4)22 with a mean HSI (SD) of 2.17 (1.38).
A total of 252 (66.8%) parents were willing to set a quit date and interested in receiving NRT. Compared to parents who were not interested in NRT (n=125; 33.2%), interested parents were more likely to: be older with mean (SD) parent age 33.6 (8.2) vs 31.9 (8.2) years; have older mean (SD) child age 5.5 (5.0) vs 4.2 (4.6) years; be non-Hispanic Black (54% vs 49.6%), and have higher motivation to quit [mean (SD) 7.0 (2.4) vs. 5.2 (2.6)]. Of the parents with a child being evaluated in UC (65.2%), the majority were interested in NRT (70.6% vs 54.5%) (Table 1). Additionally, more parents with a child being evaluated in UC were interested in NRT compared to parents with a child being evaluated in the PED (72.4% compared to 56.5%; p=0.002).
Table 1
Parent characteristics overall and by interest in setting a quit date and receiving free NRT
| Characteristics | Overall (N=377) | Interested in NRT (N=252) | Not interested in NRT (N=125) | p* |
|---|---|---|---|---|
| Parent age (years) | 0.02 | |||
| Mean (SD) | 33.1 (8.2) | 33.6 (8.2) | 31.9 (8.2) | |
| Median (IQR) | 31.0 (27.0–37.0) | 32.0 (28.0–38.0) | 30.0 (26.0–35.5) | |
| Range | 20.0–64.0 | 20.0–64.0 | 20.0–64.0 | |
| Child age (years) | 0.008 | |||
| Mean (SD) | 5.1 (4.9) | 5.5 (5.0) | 4.2 (4.6) | |
| Median (IQR) | 3.3 (1.1–8.1) | 3.9 (1.3–9.0) | 2.2 (0.7–6.6) | |
| Range | 0–17.8 | 0–17.8 | 0–17.3 | |
| Parent sex, n (%) | ||||
| Female | 330 (87.5) | 215 (85.3) | 115 (92.0) | 0.06 |
| Parent race, n (%) | 0.01 | |||
| Black, non-Hispanic | 198 (52.5) | 136 (54.0) | 62 (49.6) | |
| White, non-Hispanic | 149 (39.5) | 93 (36.9) | 56 (44.8) | |
| Hispanic | 8 (2.1) | 3 (1.2) | 5 (4.0) | |
| Other | 22 (5.8) | 20 (7.9) | 2 (1.6) | |
| Insurance type, n (%) | 0.65 | |||
| Public/self-pay | 324 (85.9) | 218 (88.5) | 106 (84.8) | |
| Commercial | ||||
| Income, n (%) | 0.74 | |||
| ≤5000 | 144 (38.2) | 98 (38.9) | 46 (36.8) | |
| 5001–15000 | 87 (23.1) | 53 (21.0) | 34 (27.2) | |
| 15001–30000 | 88 (23.3) | 62 (24.6) | 26 (20.8) | |
| 30001–50000 | 40 (10.6) | 28 (11.1) | 12 (9.6) | |
| >50001 US$ | 18 (4.8) | 11 (4.4) | 7 (5.6) | |
| Financial strain | 0.07 | |||
| Mean (SD) | 2.46 (1.10) | 2.53 (1.10) | 2.33 (1.10) | |
| Median (IQR) | 2.33 (1.67–3.33) | 2.33 (1.67–3.33) | 2.00 (1.33–3.00) | |
| Range | 1.00–5.00 | 1.00–5.00 | 1.00–5.00 | |
| Unemployed, n/N (%) | 162/375 (43.2) | 103/250 (41.2) | 59/125 (47.2) | 0.27 |
| Education, n (%) | 0.23 | |||
| Less than HS | 67 (17.8) | 39 (15.5) | 28 (22.4) | |
| Completed HS | 153 (40.6) | 105 (41.7) | 48 (38.4) | |
| Vocational school | 157 (41.6) | 108 (42.9) | 49 (39.2) | |
| Some College and beyond | ||||
| Cigarettes/day | 0.89 | |||
| Mean (SD) | 11.5 (7.1) | 11.3 (6.6) | 11.8 (7.9) | |
| Median (IQR) | 10.0 (6.0–15.0) | 10.0 (6.0–15.0) | 10.0 (6.0–15.0) | |
| Range | 1.0–40.0 | 1.0–40.0 | 2.0–40.0 | |
| Prior quit attempts, n (%) | 0.20 | |||
| 0 | 116 (30.8) | 71 (28.2) | 45 (36.0) | |
| 1 | 78 (20.7) | 51 (20.2) | 27 (21.6) | |
| ≥2 | 183 (48.5) | 130 (51.6) | 53 (42.4) | |
| HSI | 0.58 | |||
| Mean (SD) | 2.17 (1.38) | 2.19 (1.35) | 2.11 (1.44) | |
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | |
| Range | 0.0–6.0 | 0.0–6.0 | 0.0–6.0 | |
| Readiness to quit | <0.001 | |||
| Mean (SD) | 6.40 (2.59) | 6.98 (2.38) | 5.23 (2.61) | |
| Median (IQR) | 6.0 (5.0–8.0) | 7.0 (6.0–9.0) | 5.0 (4.0–7.0) | |
| Range | 0.0–10.0 | 0.0–10.0 | 0.0–10.0 | |
| Patient in UC, n (%) | 246 (65.3) | 178 (70.6) | 68 (54.4) | 0.002 |
Screening for NRT eligibility
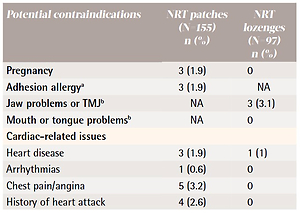
After assessing willingness to set a quit date in 14 days and interest in receiving free NRT, of the 252 parents that were asked, 155 preferred NRT patches and 97 preferred lozenges. Parents were then screened with a modified version of the U.S. Food and Drug Administration (FDA)-approved contraindications for NRT18,19 created for this study. We used the strictest criteria for NRT approval given the literature that reports that pediatricians are uncomfortable prescribing cessation medications to adults who are not their direct patients5. Table 2 gives the checklist and frequencies of contraindications for NRT. A total of 53 (21.0%) interested parents were excluded from receiving NRT because they had at least one contraindication with the top two being hypertension (n=20; 7.9%) and cardiac-related issues (n=14; 5.6%).
Table 2
Contraindication checklist and frequency of NRT ineligibility for first product choice by interested parents (N=252)
| Potential contraindications | NRT patches (N=155) n (%) | NRT lozenges (N=97) n (%) |
|---|---|---|
| Pregnancy | 3 (1.9) | 0 |
| Adhesion allergya | 3 (1.9) | NA |
| Jaw problems or TMJb | NA | 3 (3.1) |
| Mouth or tongue problemsb | NA | 0 |
| Cardiac-related issues | ||
| Heart disease | 3 (1.9) | 1 (1) |
| Arrhythmias | 1 (0.6) | 0 |
| Chest pain/angina | 5 (3.2) | 0 |
| History of heart attack | 4 (2.6) | 0 |
| Psoriasis or dermatitisa | 1 (0.6) | 0 |
| Active peptic ulcers or esophagitis | 0 | 0 |
| Severe kidney problems | 1 (0.6) | 0 |
| Hypertensionc | 16 (10.3) | 4 (4.1) |
| Hyperthyroidism | 0 | 1 (1) |
| Pheochromocytoma | 0 | 0 |
| Diabetes mellitus | 1 (0.6) | 6 (6.2) |
Dosing for NRT
Parents who were screened as eligible for NRT and who preferred NRT patches received a 6-week supply of the 21 mg patch if they smoked >20 cigarettes per day; otherwise they were given the 14 mg patch. Eligible parents who preferred NRT lozenges received a 6-week supply of the 4 mg lozenge if they smoked their first cigarette within 30 minutes of waking up; otherwise they were given the 2 mg lozenge.
Receipt and subsequent use of NRT
Of the 199 NRT eligible parents, 100% received NRT during their child’s emergency visit. A total of 129 (65%) received patches: 68 (53%) received the 21 mg patch and the remainder received the 14 mg patch. Seventy (35%) received lozenges: 48 (69%) received the 4 mg lozenge and the remainder received the 2 mg lozenge.
Home visits were successfully conducted on 119 (59.8%) parents who received NRT during their child’s emergency visit. Of those, 94 (79.0%) reported they used at least some of the NRT and 65 (54.6%) used at least 80% of their original supply, and 50 (53.2%) requested an additional 6-week supply. A total of 22 (44%) participants requested to have their NRT modality changed at the 6-week time point: 13 changed from the NRT patch to lozenge, and 9 changed from the NRT lozenge to patch. No serious adverse events were reported among parents. Only 5/94 (5.3%) participants reported NRT patchrelated minor side effects: rash (n=3), pruritis (n=1), and nausea (n=1).
DISCUSSION
Despite the knowledge that NRT is effective in increasing abstinence in smokers, few pediatric healthcare providers prescribe NRT to parents5-7. This study is the first to provide free NRT to parental smokers during their child’s (and not the parents’) emergency visit. In a previous PED-based project, we conducted a similar study in which parents were given a voucher for a free 2-week NRT supply that they had to redeem at the hospital pharmacy23. Although 92% of eligible parents expressed interest in receiving the voucher, only 47% of eligible parents redeemed the voucher and usage rate was not obtained. In contrast, only 67% of parents in the present study were interested but 100% of these parents who had no contraindications received NRT during their child’s emergency and 79% reported some use. Since NRT is the preferred choice of cessation medication of many smokers24,25, our findings suggest that the provision of free NRT is acceptable to adult smokers in the emergency setting. Research in the adult in-patient setting has found that the provision of free NRT results in increased rates of subsequent use during hospitalization and after discharge26,27. The high rate of NRT usage after the emergency visit reported in our study parallels these findings and is encouraging.
Previous studies have provided free NRT to smokers in the adult ED. Overall, these studies have demonstrated increased periods of abstinence and increased quit attempts in those who were given NRT9,13-16. Before evaluating the success of NRT on abstinence among parental smokers in the emergency setting, pediatric healthcare providers and researchers need guidance on which parents may be more receptive to receiving NRT and the potential associated side effects of NRT. This knowledge may potentially increase providers’ confidence and comfort in providing NRT to adults who are not their patients. Based on our results, parents in the UC setting and parents who are older, Black, and more motivated to quit, may be more receptive to receiving free NRT. While the differences in parent and child age were statistically significant, the differences may not be clinically meaningful as the relatively small actual difference in age may not help to guide decisions on which parents are likely to be interested in NRT. However, the finding that parents who had a child in the UC were more interested in NRT, compared to parents who had a child in the PED, was more robust and may help to guide the provision of NRT in a clinical setting. A possible explanation for this is that when a child is in the PED, parents may be more concerned about their child’s illness than they are about quitting smoking compared to when a child is in the UC and not as sick. The PED setting may also be larger, busier and more overwhelming to parents whereas the UC environment may be perceived as more similar to an outpatient clinic. This finding suggests that the administration of free NRT may be received better in the UC setting, but further research to explore the reasons for interest in NRT is warranted. Similar to other research24,28,29, we also found that parents who were Black or more motivated to quit were more receptive to cessation efforts. These findings are clinically relevant to decision-making about NRT. Clinicians and researchers may want to focus their efforts on interventions for Black smokers and/or develop methods for assessing motivation to quit that fit into the PED and UC work flows. Although other research has shown that lower income groups were not receptive to cessation interventions24,30-32, we did not observe any differences in interest in NRT with respect to either income or education. Moreover, we did not observe higher rates of interest in parents with higher nicotine dependence as seen in other studies24,33, but this is most likely due to the overall lower levels of nicotine dependence observed in our sample.
Using strict criteria to identify parents with potential contraindications to NRT, we found that 21% were ineligible. However, certain criteria such as hypertension are usually not considered contraindications in real world adult settings18,19,34. Thus, more parents would likely be eligible to receive NRT in the emergency setting. For example, if we eliminated hypertension as a contraindication, then only 13% of parents would have been considered ineligible for NRT. Additionally, similar to work that reports low rates of side effects of NRT8,35, parents in this study reported only minor expected side effects. The lack of serious side effects in screened participants underscores the safety of providing NRT (which is available over-the-counter) in the emergency setting.
Limitations
There are limitations that should be considered when interpreting our results. The study sample was drawn from a population of smokers who presented with their children to a tertiary care PED or UC at a single Midwestern children’s hospital, which limits generalizability. This population was predominantly from households with low socioeconomic status, which also limits generalizability. We were unable to follow up with all parents who received NRT at 6 weeks so rates of subsequent usage may have been lower or higher than reported.
CONCLUSIONS
This study provides important information on the interest of receiving free NRT, the feasibility of providing NRT, and the safety of using NRT among parental smokers in the emergency setting. We found that parental smokers who were older, had older children, were non-Hispanic Black, had a higher readiness to quit, and who had a child being evaluated in the UC, as opposed to the PED, were more interested in receiving NRT. Since all parental smokers need to be assisted in quitting smoking, finding ways to engage all smokers in quit attempts at every pediatric healthcare visit should be further examined and prioritized in future studies.